Volume 14, Issue 6 (Nov & Dec 2024)
J Research Health 2024, 14(6): 587-592 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Faroughi F, Modanloo M, Sadeghi M, Khodabakhshi B, Taghizadeh Firozjaie I. Determination of D-dimer Levels in Patients Who Survived and Died From COVID-19: A Case-control Study. J Research Health 2024; 14 (6) :587-592
URL: http://jrh.gmu.ac.ir/article-1-2337-en.html
URL: http://jrh.gmu.ac.ir/article-1-2337-en.html
Forough Faroughi1 

 , Mahnaz Modanloo2
, Mahnaz Modanloo2 

 , Mohammad Sadeghi3
, Mohammad Sadeghi3 

 , Behnaz Khodabakhshi4
, Behnaz Khodabakhshi4 

 , Iman Taghizadeh Firozjaie5
, Iman Taghizadeh Firozjaie5 




 , Mahnaz Modanloo2
, Mahnaz Modanloo2 

 , Mohammad Sadeghi3
, Mohammad Sadeghi3 

 , Behnaz Khodabakhshi4
, Behnaz Khodabakhshi4 

 , Iman Taghizadeh Firozjaie5
, Iman Taghizadeh Firozjaie5 


1- Student Research Committee, Kashan University of Medical Sciences, Kashan, Iran.
2- Department of Nursing, Nursing Research Center, School of Nursing and Midwifery, Golestan University of Medical Sciences, Gorgan, Iran.
3- Department of Medical-Surgical Nursing, School of Nursing and Midwifery, Golestan University of Medical Sciences, Gorgan, Iran.
4- Department of Infectious Diseases Research, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran.
5- Department of Medical-Surgical Nursing, School of Nursing and Midwifery, Golestan University of Medical Sciences, Gorgan, Iran. ,imantaghizade011@gmail.com
2- Department of Nursing, Nursing Research Center, School of Nursing and Midwifery, Golestan University of Medical Sciences, Gorgan, Iran.
3- Department of Medical-Surgical Nursing, School of Nursing and Midwifery, Golestan University of Medical Sciences, Gorgan, Iran.
4- Department of Infectious Diseases Research, School of Medicine, Golestan University of Medical Sciences, Gorgan, Iran.
5- Department of Medical-Surgical Nursing, School of Nursing and Midwifery, Golestan University of Medical Sciences, Gorgan, Iran. ,
Full-Text [PDF 535 kb]
(495 Downloads)
| Abstract (HTML) (3030 Views)
Full-Text: (874 Views)
Introduction
The virus responsible for COVID-19, SARS-CoV-2, is a single-stranded RNA virus belonging to the beta-coronavirus family [1]. SARS-CoV-2 is a new generation of this family that was discovered in 2019 and spread rapidly around the world; this virus had not been identified in humans prior to that time [2]. By the end of March 2020, 600,000 patients worldwide had been diagnosed with a positive coronavirus test [3]. Studies have shown that patients with COVID-19 experience mild infections, but some individuals may develop severe infections, leading to increased hospitalization and mortality. The virus infects host cells using its surface protein (S), which binds to angiotensin-converting enzyme 2 (ACE2). ACE2 is a membrane-bound peptidase expressed in the heart, lungs, gastrointestinal tract and kidneys, playing an important role in immune pathways [4-6]. Common symptoms of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, acute respiratory syndrome, kidney failure and even death [7-9].
Standard recommendations to prevent the spread of infection include washing hands regularly, covering the mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs and avoiding close contact with people who have respiratory symptoms, such as coughing and sneezing [10]. Most co-morbidities in COVID-19 patients are hypertension, diabetes, heart disease, and chronic obstructive pulmonary disease (COPD) [11].
Studies have shown that changes in blood coagulation, as well as an increase in D-dimer levels, occur in patients with COVID-19 [12, 13]. D-dimer, a breakdown product of fibrin, is a relatively small protein fragment that is present in the blood following the destruction of blood clots by fibrinolysis. The determination of D-dimer concentration is a test used to diagnose thrombotic conditions, including pulmonary embolism and disseminated intravascular coagulation (DIC) [14]. Changes in coagulation factors, such as D-dimer, are among the factors that increase the hospitalization and mortality of patients [15, 16]. Some laboratory abnormalities include decreased white blood cell and lymphocyte counts, neutrophilia, thrombocytopenia, increased C-reactive protein (CRP), elevated erythrocyte sedimentation rate (ESR) and abnormal procalcitonin (PCT) levels in most patients [17].
Although the role of D-dimer in patients with COVID-19 is not fully understood, studies have shown that patients with COVID-19 who have high levels of D-dimer may develop acute hemorrhagic encephalopathy and ischemic stroke [18, 19]. The threshold value of D-dimer to predict in-hospital mortality was 2.0 μg/mL, with a sensitivity of 92.3% and a specificity of 83.3%. Among the patients, 67 had D-dimer levels ≥2.0 μg/mL, while 267 had levels <2 μg/mL upon admission. Thirteen deaths occurred during hospitalization. Patients with D-dimer levels ≥2 μg/mL had a higher incidence of mortality compared to those with D-dimer levels <2 μg/mL [3]. Therefore, an increase in D-dimer levels in COVID-19 patients is useful for the rapid identification of high disease severity, pulmonary complications, and the risk of venous thromboembolism. This information can assist in risk stratification and the early introduction of therapeutic interventions that may reduce morbidity and mortality from COVID-19. Considering the role of D-dimer in increasing the mortality of patients with COVID-19, this study was conducted to determine the levels of D-dimer in patients who survived and those who died from COVID-19.
Methods
Study design and participants
This case-control study was conducted retrospectively at 5th Azar and Sayad Shirazi hospitals, which serve as referral hospitals for COVID-19 patients affiliated with the Golestan University of Medical Sciences, from March 20, 2020, to June 20, 2021.
On consecutive days, the researchers reviewed the medical records of all patients with COVID-19 at the selected hospitals and identified patients who met the inclusion criteria for data extraction.
Inclusion criteria included being at least 18 years old, hospitalization due to COVID-19 with a specialized diagnosis, a positive chest radiograph or PCR test and a D-dimer test performed in the patient’s file. Exclusion criteria included pregnancy, cancer, hematologic malignancy, chronic liver disease, acute coronary syndrome, and surgery or trauma within the past 30 days. Cases, in which some patient record information was incomplete were excluded from the study.
Out of the 158 patient files eligible for the study, 107 patients were assigned to the survival group and 51 patients to the death group. Based on the outcome of the disease, the samples were classified into two groups: Those who recovered from COVID-19 (control group) and those who died due to COVID-19 (case group). The D-dimer levels (exposure) were investigated for the presence or absence of a relationship with the increased risk of death due to COVID-19, hospitalization history, drug history, and drug abuse. The items raised in the research exclusion criteria could be considered confounding factors in the study; therefore, they were treated as exceptions and removed.
Statistical analysis
The normality of continuous measurements was assessed, and results were expressed as Mean±SD. Categorical variables were expressed as numbers (percentages). A logistic regression analysis was performed between the case and control groups, and the odds ratio was reported. We used SPSS software, version 21 for all analyses, and two-tailed P<0.05 were considered statistically significant.
Results
A total of 158 eligible samples were included in this study, of whom 43% were men and 57% were women in the survivor group, while 56.9% were men and 43.1% were women in the death group. The average age of the samples in the survival and death groups was 55.64±17.41 and 64.49±19.27 years, respectively. Table 1 shows the basic clinical characteristics of the patients, including age, gender, chronic disease, history of hospitalization, history of drug use, and drug use on admission.

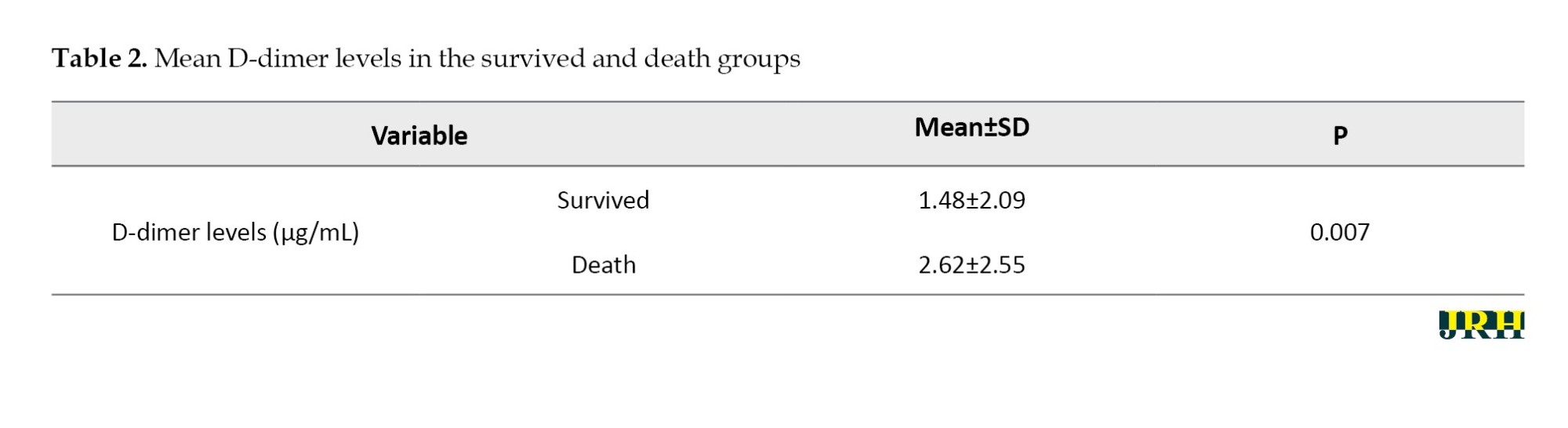
The mean D-dimer levels in the surviving group was 1.48±2.09 µg/mL,while the death group showed mean D-dimer levels of 2.62±2.55 µg/mL, (P=0.007; Table 2), and the two groups were significantly different regarding D-dimer levels.
Based on the fitted model given in Table 3, the significance of the Wald test for D-dimer was <0.05 and significant. The interpretation of the odds ratio (Exp [βi]) is as follows: The chance of death due to COVID-19 in patients with high D-dimer levels is 1.29 times that of patients with lower D-dimer levels. The P for hospitalization history, drug history and drug abuse were >0.05, indicating that these variables did not significantly predict in-hospital death for patients with COVID-19.

Discussion
The main finding of this study was that D-dimer levels on admission were an independent predictor of in-hospital death for patients with COVID-19. The interpretation of the odds ratio indicated that the probability of death due to COVID-19 in patients with high D-dimer levels was 1.29 times that of patients with lower D-dimer levels. Guan et al. analyzed 1099 patients with laboratory-confirmed COVID-19 from more than 550 hospitals in China and found that D-dimer levels were significantly higher in non-survivors than in survivors [20]. Huang et al. showed that D-dimer levels on admission were higher in patients who required intensive care support than in patients who did not, with a mean level of 0.5 μg/mL [6]. This finding is consistent with the results of our research, which reported D-dimer levels in deceased patients with an average of 2.62 µg/mL and in recovered patients with an average of 1.48 µg/mL. The average D-dimer levels in deceased patients were significantly higher compared to recovered patients.
Zhang et al. suggested that for patients with significantly elevated D-dimer levels (cutoff of 2.0 μg/mL; fourfold increase), hospitalization and close monitoring should be considered, even in the absence of other severe symptoms [3]. High D-dimer has always been associated with adverse effects [21, 22]. Previously, the lack of specificity was considered a weakness of D-dimer. However, this low specificity has become one of its advantages in prognostic assessment [23]. Poudel et al. showed that the mean D-dimer levels among patients who survived were 1.067±1.705 μg/mL, compared to 3.208±2.613 μg/mL among patients who died. This represents a statistically significant difference (P<0.001, independent samples t-test). This study found that higher D-dimer levels at hospitalization were significantly associated with in-hospital mortality in COVID-19 patients [24].
Unlike the present study, several other studies found that D-dimer levels were not related to the severity of the disease, showing no significant difference in D-dimer levels between patients with severe and mild forms of COVID-19. The difference between the results of the present study and the findings of these studies can be due to the difference in the sample sizes. In the study by Lui et al. the number of patients with severe disease was 7; in the study by Qian et al. it was 9 and in the study by Jian et al. it was 13. Therefore, these studies had limited statistical power to investigate the relationship between dimer-D levels and mortality and disease severity [25-27]. On the other hand, the results of a series of studies showed that an increase in D-dimer levels at the onset of hospitalization in patients with COVID-19 infection is associated with an increased risk of severe disease progression and death [28-30].
A systematic review published in August 2020 found that COVID-19 patients with elevated d-dimer levels were at increased risk of severe disease and mortality, noting that no cutoff value had been defined to predict adverse events [31]. A retrospective study in the US involving 1065 hospitalized patients found that each 1 μg/mL increase in D-dimer levels during hospitalization was associated with a hazard ratio of 1.06 for all-cause mortality. However, D-dimer was a poor prognostic marker for predicting mortality [32].
This research had limitations. Our study may have a selection bias because it was a retrospective study. Despite our efforts to include all eligible patients, some were excluded from admission due to missing D-dimer levels in their medical records. A multi-parameter prediction model that includes D-dimer and other variables may provide better predictive capabilities for COVID-19 patients.
Conclusion
The D-dimer levels on admission are an accurate biomarker for predicting mortality in patients with COVID-19. Therefore, D-dimer levels can be an easy and cheap laboratory indicator for the prognosis of COVID-19. Monitoring D-dimer levels will be a crucial approach in the clinical management of COVID-19 infection.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Golestan University of Medical Sciences (Code: IR.GOUMS.REC.1400.243) and in coordination with the treating physicians. All information was kept confidential by the researcher, and no interventions were made on patients. All other procedures adhered to the standards of Golestan University of Medical Sciences.
Funding
This study was financially supported by Golestan University of Medical Sciences.
Authors' contributions
Study design: Forough Faroughi, Mahnaz Modanloo and Iman Taghizadeh Firozjaie; Data collection: Mohammad Sadeghi, Forough Faroughi and Iman Taghizadeh Firozjaie; Data analyses: Mahnaz Modanloo, Forough Faroughi, Behnaz Khodabakhshi and Iman Taghizadeh Firozjaie; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate all members of the support group of the 5th Azar and Sayad Shirazi hospitals affiliated with the Golestan University of Medical Sciences.
References
The virus responsible for COVID-19, SARS-CoV-2, is a single-stranded RNA virus belonging to the beta-coronavirus family [1]. SARS-CoV-2 is a new generation of this family that was discovered in 2019 and spread rapidly around the world; this virus had not been identified in humans prior to that time [2]. By the end of March 2020, 600,000 patients worldwide had been diagnosed with a positive coronavirus test [3]. Studies have shown that patients with COVID-19 experience mild infections, but some individuals may develop severe infections, leading to increased hospitalization and mortality. The virus infects host cells using its surface protein (S), which binds to angiotensin-converting enzyme 2 (ACE2). ACE2 is a membrane-bound peptidase expressed in the heart, lungs, gastrointestinal tract and kidneys, playing an important role in immune pathways [4-6]. Common symptoms of infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. In more severe cases, infection can cause pneumonia, acute respiratory syndrome, kidney failure and even death [7-9].
Standard recommendations to prevent the spread of infection include washing hands regularly, covering the mouth and nose when coughing and sneezing, thoroughly cooking meat and eggs and avoiding close contact with people who have respiratory symptoms, such as coughing and sneezing [10]. Most co-morbidities in COVID-19 patients are hypertension, diabetes, heart disease, and chronic obstructive pulmonary disease (COPD) [11].
Studies have shown that changes in blood coagulation, as well as an increase in D-dimer levels, occur in patients with COVID-19 [12, 13]. D-dimer, a breakdown product of fibrin, is a relatively small protein fragment that is present in the blood following the destruction of blood clots by fibrinolysis. The determination of D-dimer concentration is a test used to diagnose thrombotic conditions, including pulmonary embolism and disseminated intravascular coagulation (DIC) [14]. Changes in coagulation factors, such as D-dimer, are among the factors that increase the hospitalization and mortality of patients [15, 16]. Some laboratory abnormalities include decreased white blood cell and lymphocyte counts, neutrophilia, thrombocytopenia, increased C-reactive protein (CRP), elevated erythrocyte sedimentation rate (ESR) and abnormal procalcitonin (PCT) levels in most patients [17].
Although the role of D-dimer in patients with COVID-19 is not fully understood, studies have shown that patients with COVID-19 who have high levels of D-dimer may develop acute hemorrhagic encephalopathy and ischemic stroke [18, 19]. The threshold value of D-dimer to predict in-hospital mortality was 2.0 μg/mL, with a sensitivity of 92.3% and a specificity of 83.3%. Among the patients, 67 had D-dimer levels ≥2.0 μg/mL, while 267 had levels <2 μg/mL upon admission. Thirteen deaths occurred during hospitalization. Patients with D-dimer levels ≥2 μg/mL had a higher incidence of mortality compared to those with D-dimer levels <2 μg/mL [3]. Therefore, an increase in D-dimer levels in COVID-19 patients is useful for the rapid identification of high disease severity, pulmonary complications, and the risk of venous thromboembolism. This information can assist in risk stratification and the early introduction of therapeutic interventions that may reduce morbidity and mortality from COVID-19. Considering the role of D-dimer in increasing the mortality of patients with COVID-19, this study was conducted to determine the levels of D-dimer in patients who survived and those who died from COVID-19.
Methods
Study design and participants
This case-control study was conducted retrospectively at 5th Azar and Sayad Shirazi hospitals, which serve as referral hospitals for COVID-19 patients affiliated with the Golestan University of Medical Sciences, from March 20, 2020, to June 20, 2021.
On consecutive days, the researchers reviewed the medical records of all patients with COVID-19 at the selected hospitals and identified patients who met the inclusion criteria for data extraction.
Inclusion criteria included being at least 18 years old, hospitalization due to COVID-19 with a specialized diagnosis, a positive chest radiograph or PCR test and a D-dimer test performed in the patient’s file. Exclusion criteria included pregnancy, cancer, hematologic malignancy, chronic liver disease, acute coronary syndrome, and surgery or trauma within the past 30 days. Cases, in which some patient record information was incomplete were excluded from the study.
Out of the 158 patient files eligible for the study, 107 patients were assigned to the survival group and 51 patients to the death group. Based on the outcome of the disease, the samples were classified into two groups: Those who recovered from COVID-19 (control group) and those who died due to COVID-19 (case group). The D-dimer levels (exposure) were investigated for the presence or absence of a relationship with the increased risk of death due to COVID-19, hospitalization history, drug history, and drug abuse. The items raised in the research exclusion criteria could be considered confounding factors in the study; therefore, they were treated as exceptions and removed.
Statistical analysis
The normality of continuous measurements was assessed, and results were expressed as Mean±SD. Categorical variables were expressed as numbers (percentages). A logistic regression analysis was performed between the case and control groups, and the odds ratio was reported. We used SPSS software, version 21 for all analyses, and two-tailed P<0.05 were considered statistically significant.
Results
A total of 158 eligible samples were included in this study, of whom 43% were men and 57% were women in the survivor group, while 56.9% were men and 43.1% were women in the death group. The average age of the samples in the survival and death groups was 55.64±17.41 and 64.49±19.27 years, respectively. Table 1 shows the basic clinical characteristics of the patients, including age, gender, chronic disease, history of hospitalization, history of drug use, and drug use on admission.

The mean D-dimer levels in the surviving group was 1.48±2.09 µg/mL,while the death group showed mean D-dimer levels of 2.62±2.55 µg/mL, (P=0.007; Table 2), and the two groups were significantly different regarding D-dimer levels.

Based on the fitted model given in Table 3, the significance of the Wald test for D-dimer was <0.05 and significant. The interpretation of the odds ratio (Exp [βi]) is as follows: The chance of death due to COVID-19 in patients with high D-dimer levels is 1.29 times that of patients with lower D-dimer levels. The P for hospitalization history, drug history and drug abuse were >0.05, indicating that these variables did not significantly predict in-hospital death for patients with COVID-19.

Discussion
The main finding of this study was that D-dimer levels on admission were an independent predictor of in-hospital death for patients with COVID-19. The interpretation of the odds ratio indicated that the probability of death due to COVID-19 in patients with high D-dimer levels was 1.29 times that of patients with lower D-dimer levels. Guan et al. analyzed 1099 patients with laboratory-confirmed COVID-19 from more than 550 hospitals in China and found that D-dimer levels were significantly higher in non-survivors than in survivors [20]. Huang et al. showed that D-dimer levels on admission were higher in patients who required intensive care support than in patients who did not, with a mean level of 0.5 μg/mL [6]. This finding is consistent with the results of our research, which reported D-dimer levels in deceased patients with an average of 2.62 µg/mL and in recovered patients with an average of 1.48 µg/mL. The average D-dimer levels in deceased patients were significantly higher compared to recovered patients.
Zhang et al. suggested that for patients with significantly elevated D-dimer levels (cutoff of 2.0 μg/mL; fourfold increase), hospitalization and close monitoring should be considered, even in the absence of other severe symptoms [3]. High D-dimer has always been associated with adverse effects [21, 22]. Previously, the lack of specificity was considered a weakness of D-dimer. However, this low specificity has become one of its advantages in prognostic assessment [23]. Poudel et al. showed that the mean D-dimer levels among patients who survived were 1.067±1.705 μg/mL, compared to 3.208±2.613 μg/mL among patients who died. This represents a statistically significant difference (P<0.001, independent samples t-test). This study found that higher D-dimer levels at hospitalization were significantly associated with in-hospital mortality in COVID-19 patients [24].
Unlike the present study, several other studies found that D-dimer levels were not related to the severity of the disease, showing no significant difference in D-dimer levels between patients with severe and mild forms of COVID-19. The difference between the results of the present study and the findings of these studies can be due to the difference in the sample sizes. In the study by Lui et al. the number of patients with severe disease was 7; in the study by Qian et al. it was 9 and in the study by Jian et al. it was 13. Therefore, these studies had limited statistical power to investigate the relationship between dimer-D levels and mortality and disease severity [25-27]. On the other hand, the results of a series of studies showed that an increase in D-dimer levels at the onset of hospitalization in patients with COVID-19 infection is associated with an increased risk of severe disease progression and death [28-30].
A systematic review published in August 2020 found that COVID-19 patients with elevated d-dimer levels were at increased risk of severe disease and mortality, noting that no cutoff value had been defined to predict adverse events [31]. A retrospective study in the US involving 1065 hospitalized patients found that each 1 μg/mL increase in D-dimer levels during hospitalization was associated with a hazard ratio of 1.06 for all-cause mortality. However, D-dimer was a poor prognostic marker for predicting mortality [32].
This research had limitations. Our study may have a selection bias because it was a retrospective study. Despite our efforts to include all eligible patients, some were excluded from admission due to missing D-dimer levels in their medical records. A multi-parameter prediction model that includes D-dimer and other variables may provide better predictive capabilities for COVID-19 patients.
Conclusion
The D-dimer levels on admission are an accurate biomarker for predicting mortality in patients with COVID-19. Therefore, D-dimer levels can be an easy and cheap laboratory indicator for the prognosis of COVID-19. Monitoring D-dimer levels will be a crucial approach in the clinical management of COVID-19 infection.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Golestan University of Medical Sciences (Code: IR.GOUMS.REC.1400.243) and in coordination with the treating physicians. All information was kept confidential by the researcher, and no interventions were made on patients. All other procedures adhered to the standards of Golestan University of Medical Sciences.
Funding
This study was financially supported by Golestan University of Medical Sciences.
Authors' contributions
Study design: Forough Faroughi, Mahnaz Modanloo and Iman Taghizadeh Firozjaie; Data collection: Mohammad Sadeghi, Forough Faroughi and Iman Taghizadeh Firozjaie; Data analyses: Mahnaz Modanloo, Forough Faroughi, Behnaz Khodabakhshi and Iman Taghizadeh Firozjaie; Writing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate all members of the support group of the 5th Azar and Sayad Shirazi hospitals affiliated with the Golestan University of Medical Sciences.
References
- Chen L, Liu W, Zhang Q, Xu K, Ye G, Wu W, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerging Microbes & Infections. 2020; 9(1):313-9. [DOI:10.1080/22221751.2020.1725399]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020; 382(8):727-33.[DOI:10.1056/NEJMoa2001017]
- Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. Journal of Thrombosis and Haemostasis. 2020; 18(6):1324-9. [DOI:10.1111/jth.14859]
- Yaron JR, Ambadapadi S, Zhang L, Chavan RN, Tibbetts SA, Keinan S, et al. Immune protection is dependent on the gut microbiome in a lethal mouse gammaherpesviral infection. Scientific Reports. 2020; 10(1):2371. [DOI:10.1038/s41598-020-59269-9]
- Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020; 181(2):281-92. [DOI:10.1016/j.cell.2020.02.058]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020; 395(10223):497-506. [DOI:10.1016/S0140-6736(20)30183-5]
- Mahajan A, Hirsch J. Novel coronavirus: What neuroradiologists as citizens of the world need to know. American Journal of Neuroradiology. 2020; 41(4):552-4. [DOI:10.3174/ajnr.A6526]
- World Health Organization (WHO). COVID-19: Operational guidance for maintaining essential health services during an outbreak: Interim guidance, 25 March 2020. Geneva: World Health Organization; 2020. [Link]
- Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents. 2020; 55(3):105924. [DOI:10.1016/j.ijantimicag.2020.105924]
- World Health Organization (WHO). Coronavirus disease: What you need to know [Internet]. 2020 [Updated 2020 March 9]. Available from: [Link]
- Mubarak AR, Esa T, Widaningsih Y, Bahrun U. D-dimer analysis in COVID-19 patients. Indonesian Journal of Clinical Pathology and Medical Laboratory. 2021; 28(1):5-9. [DOI:10.24293/ijcpml.v28i1.1812]
- Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. Journal of Intensive Care. 2020; 8(49):1-11. [DOI:10.1186/s40560-020-00466-z]
- Snijders D, Schoorl M, Schoorl M, Bartels PC, van der Werf TS, Boersma WG. D-dimer levels in assessing severity and clinical outcome in patients with community-acquired pneumonia. A secondary analysis of a randomised clinical trial. European Journal of Internal Medicine. 2012; 23(5):436-41. [DOI:10.1016/j.ejim.2011.10.019]
- Olson JD. D-dimer: An overview of hemostasis and fibrinolysis, assays, and clinical applications. Advances in Clinical Chemistry. 2015; 69:1-46. [DOI:10.1016/bs.acc.2014.12.001]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet. 2020; 395(10223):507-13. [DOI:10.1016/S0140-6736(20)30211-7]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. The Lancet. 2020; 395(10229):1054-62. [DOI:10.1016/S0140-6736(20)30566-3]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020; 323(13):1239-42. [DOI:10.1001/jama.2020.2648]
- Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology. 2020; 296(2):E119-20. [DOI:10.1148/radiol.2020201187]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology. 2020; 77(6):683-90. [DOI:10.1001/jamaneurol.2020.1127]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020; 382(18):1708-20. [DOI:10.1056/NEJMoa200203]
- Zhang L, Zheng X, Long Y, Wu M, Chen Y, Yang J, et al. D-dimer to guide the intensity of anticoagulation in Chinese patients after mechanical heart valve replacement: A randomized controlled trial. Journal of Thrombosis and Haemostasis. 2017; 15(10):1934-41. [DOI:10.1111/jth.13782]
- Tripodi A. D-dimer testing in laboratory practice. Clinical Chemistry. 2011; 57(9):1256-62. [DOI:10.1373/clinchem.2011.166249]
- Zhang L, Long Y, Xiao H, Yang J, Toulon P, Zhang Z. Use of D-dimer in oral anticoagulation therapy. International Journal of Laboratory Hematology. 2018; 40(5):503-7. [DOI:10.1111/ijlh.12864]
- Poudel A, Poudel Y, Adhikari A, Aryal BB, Dangol D, Bajracharya T, et al. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. Plos One. 2021; 16(8):e0256744. [DOI:10.1371/journal.pone.0256744]
- Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020; 55:102763. [DOI:10.1016/j.ebiom.2020.102763]
- Liu L, Gao JY, Hu WM, Zhang XX, Guo L, Liu CQ, et al. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. MedRxiv. 2020; 2020 [Unpublished]. [DOI:10.1101/2020.02.20.20025536]
- Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. QJM. 2020; 113(7):474-81. [DOI:10.1093/qjmed/hcaa089]
- Nugroho J, Wardhana A, Maghfirah I, Mulia EPB, Rachmi DA, A’yun MQ, et al. Relationship of D-dimer with severity and mortality in SARS-CoV-2 patients: A meta-analysis. International Journal of Laboratory Hematology. 2021; 43(1):110-5. [DOI:10.1111/ijlh.13336]
- Stefanopol IA, Miulescu M, Baroiu L, Anghele A-D, Danila DM, Tiron Z. An unusual case of Meckel diverticulitis misdiagnosed as an infected urachal cyst. Medicina. 2021; 57(5):495. [DOI:10.3390/medicina57050495]
- Vidali S, Morosetti D, Cossu E, Luisi MLE, Pancani S, Semeraro V, et al. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: A systematic review. ERJ Open Research. 2020; 6(2):00260-2020. [DOI:10.1183/23120541.00260-2020]
- Shah S, Shah K, Patel SB, Patel FS, Osman M, Velagapudi P, et al. Elevated D-dimer levels are associated with increased risk of mortality in coronavirus disease 2019: A systematic review and meta-analysis. Cardiology in Review. 2020; 28(6):295-302. [DOI:10.1097/CRD.0000000000000330]
- Naymagon L, Zubizarreta N, Feld J, van Gerwen M, Alsen M, Thibaud S, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thrombosis Research. 2020; 196:99-105. [DOI:10.1016/j.thromres.2020.08.032]
Type of Study: Orginal Article |
Subject:
● International Health
Received: 2023/04/15 | Accepted: 2023/12/9 | Published: 2024/10/28
Received: 2023/04/15 | Accepted: 2023/12/9 | Published: 2024/10/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |