Volume 14, Issue 6 (Nov & Dec 2024)
J Research Health 2024, 14(6): 575-586 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rezapour M, Khanjani N, Sharafkhani R, Moosazadeh M. Multimorbidity Patterns and Their Relationship With ICU Admission and Mortality Rates in Hospitalized Patients With COVID-19 in Northern Iran. J Research Health 2024; 14 (6) :575-586
URL: http://jrh.gmu.ac.ir/article-1-2432-en.html
URL: http://jrh.gmu.ac.ir/article-1-2432-en.html
1- Department of Paramedicine, Amol School of Paramedical Sciences, Mazandaran University of Medical Sciences, Sari, Iran.
2- Neurology Research Center, Kerman University of Medical Sciences, Kerman, Iran.
3- Department of Public Health, Khoy University of Medical Sciences, Khoy, Iran.
4- Non-Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,mmoosazadeh1351@gmail.com
2- Neurology Research Center, Kerman University of Medical Sciences, Kerman, Iran.
3- Department of Public Health, Khoy University of Medical Sciences, Khoy, Iran.
4- Non-Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,
Full-Text [PDF 726 kb]
(470 Downloads)
| Abstract (HTML) (2885 Views)
Full-Text: (775 Views)
Introduction
The World Health Organization (WHO) announced the coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, as a pandemic in March 2020. It has spread rapidly in countries and territories and affected the lives and health of people worldwide. Although significant advances have been made in developing vaccines [1] and planning health measures [2] to prevent the disease and more effective therapies have been suggested, it is still important to identify potential factors affecting the severity, prognosis, and mortality of COVID-19. Additionally, effective triage for the treatment and management of patients is essential to allocate healthcare resources appropriately [3].
Epidemiological studies have shown that COVID-19 patients with chronic diseases experience higher hospitalization rates, increased disease severity, more intensive care unit (ICU) admissions, and ultimately higher mortality from COVID-19 [4-8]. Although some studies have examined the association between multimorbidity and mortality, several limitations exist in previous research. First, most prior studies have considered the comorbidity of chronic diseases separately [4-8]. This may lead to an inaccurate effect size, as many patients have multiple chronic diseases simultaneously. Therefore, considering multimorbidity (defined as the co-occurrence of at least two chronic conditions) in COVID-19 patients can provide better results for the identification of high-risk groups and informing treatment [9]. Second, while there is no consensus on the method used to measure multimorbidity, most previous studies have relied on counting the number of chronic comorbidities [5, 10-12], employing variable-centered approaches [13], or using specific cut points such as the Charlson comorbidity Index [14].
Latent class analysis (LCA), as a person-oriented grouping approach, is used to identify multimorbidity patterns [15]. In this method, patients are classified into latent clusters, which are homogeneous groups of individuals with regard to the multimorbidity of chronic diseases. In a previous study in Iran, five patterns of multimorbidity were found in the general population [15]. However, there is still no study on multimorbidity patterns among COVID-19 patients in Iran or other countries. Third, most previous studies have used relatively small sample sizes and have been based on single-center observations or have not accounted for hospital-level variations [4-6], when examining the associations between comorbidity (or multimorbidity) and outcomes, such as ICU admission and death. Hospital-level variations may be due to the hospital readiness dimensions of hospital readiness (such as incident management systems, coordination, information management, logistics, finance and administration, detection, diagnosis, isolation, case management and prevention and infection control) [16], the catchment area, the reputation of the hospitals and their physicians, tourist areas, and religious sites in the cities where the hospitals are located, among other factors. Finally, during the early phases of the COVID-19 pandemic, some general challenges faced by regions, including Mazandaran, could include issues related to healthcare infrastructure capacity due to the floating population caused by passengers, testing availability, public compliance with safety measures, and the overall management of the pandemic. Therefore, considering these challenges, this study investigated the patterns of multimorbidity among Iranian COVID-19 patients and their relation to ICU admission and death. The study opens avenues for further research, encouraging a deeper exploration of how to identify groups at risk for COVID-19 outcomes based on comorbidities and individual factors, as well as the development of more effective strategies for managing and treating patients.
Methods
Study population and design
In this retrospective cohort study, data were obtained from the surveillance system of the Medical Care Monitoring Center of the Ministry of Health and Medical Education (MOHME) of Iran, which has mandated all hospitals to register patients admitted with a diagnosis of confirmed or suspected COVID-19. The data of 13,960 COVID-19 patients from 42 hospitals in Mazandaran Province in northern Iran were collected according to the census between March 20, 2020 and July 20, 2021. These patients were hospitalized through three sources: direct visits to the hospital, referrals from outpatient centers (healthcare service centers) and referrals from other hospitals not designated for COVID-19 treatment. The inclusion criterion consisted of all positive COVID-19 patients admitted to the hospitals by RT-PCR during the specified time period, while the exclusion criterion included outpatients.
Measurement of chronic diseases
The included variables in this study were demographic characteristics (such as age and gender), the presence of chronic diseases as predictors, and ICU admission and/or death as outcomes. The chronic diseases included chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), diabetes, chronic kidney disease (CKD), chronic liver disease, malignancy, immune system diseases, chronic neurological disease, hypertension, asthma, metabolic diseases (hyper or hypo-thyroid and hyperlipidemia) and other diseases (rheumatoid arthritis and musculoskeletal diseases). These data were compiled based on the checklist prepared by the disease management center of the Ministry of Health and Medical Education, which was completed by each hospital or medical center upon admitting patients. The response options for each underlying chronic disease and for admission to the ICU and death were yes/no. Information about the morbidity of the patients was based on their medical files. Incomplete cases were addressed by reviewing the case files or through self-reporting from patients or their companions. If access to any of these sources was unavailable, the individual was excluded from the study.
Data analysis
Descriptive statistics were reported for all variables. LCA was used to extract multimorbidity patterns (grouping homogeneous patients in a class) based on the presence of different diseases. Then, we evaluated the relationship between each of these classes (or patterns) and the outcomes of COVID-19 (ICU admission and death) by adjusting age and gender via logistic regression analysis. The regression analyses were conducted using a multilevel method to account for the dependence of responses observed among patients belonging to the same hospital and to correct for its effects. LCA was conducted in an exploratory and iterative process with an increasing number of classes. The optimal number of classes was identified based on model fit indices in combination with empirical evidence and interpretability. Model fit was assessed using the Akaike information criterion (AIC), Bayesian information criterion (BIC), sample size adjusted bayesian information criterion (aBIC), the Lo–Mendell–Rubin likelihood ratio (LMR LR) test, and Vuong-Lo-Mendell-Rubin likelihood ratio (VLMR) Test. Smaller values of AIC, BIC, and aBIC indicated a better model fit [17]. The LMR is a likelihood ratio test that compares the estimated model to model to a model with one fewer class (k-1). If the LMR is not significant, the model fit will not improve by including an additional class in the model [18]. The VLMR, P are interpreted in the same manner as those of the LMR LR test. Entropy was used to assess the quality of member classification; a value closer to 1.0 indicates better classification [19].
Two-level logistic regression analysis (random intercept) was used to explore the association between each independent variable and the outcome variables (death and ICU admission) in univariate analysis. Given the clustering nature of the data, two-level (random intercept) logistic regression modeling was used, as patients were nested within hospitals. We then fitted multilevel logistic regression with the multimorbidity patterns obtained from LCA as independent variables and ICU admission (model 1) and death (model 2) as outcomes. In both models, age groups and gender were adjusted for confounders. Before running models 1 and 2, initially, a null or unconditional model was built to calculate the intra-class correlation coefficient (ICC) of hospitals. Data preparation and regression models were done in STATA software, version 16, and LCA was run in Mplus software, version 7.4.
Results
The descriptive statistics of all variables are shown in Table 1. Among the COVID-19 patients, 28.2% had one comorbidity, while 9.3% had two comorbidities, and 1.6% had three or more comorbidities. Additionally, 50.6% of patients were female and 20.1% were aged over 71. The most common comorbidities among COVID-19 patients were diabetes (20.6%) and hypertension (9.9%). The relative frequency of hospitalization for COVID-19 patients in the ICU and mortality from the disease was approximately 17% and 13%, respectively.

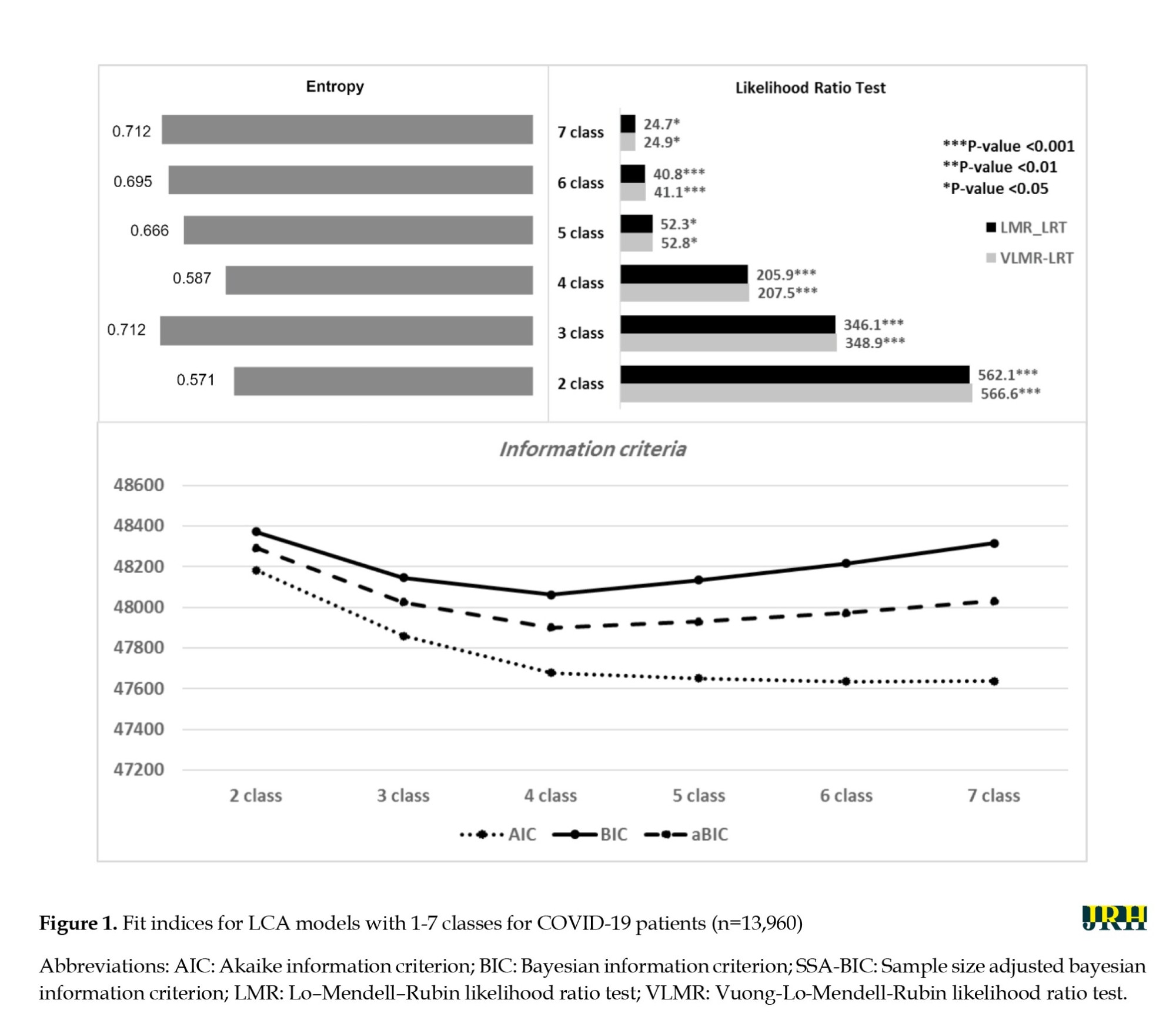
To extract multimorbidity patterns of chronic diseases, a series of latent class models were run, in which the number of classes ranged from 2 to 7. Figure 1 shows the goodness-of-fit indices for each model. The VLMR and LMR tests yielded significant P in all models; therefore, we could not use these indices as differentiators. The 4-class model was selected because the BIC and SSA-BIC values were smaller than other models, although the entropy was also lower than that of the others.
The probability of each chronic disease in the four classes (the conditional probabilities for each of the classes) is presented in Table 2. class 1, which constituted 3.7% of the patients, w::::::as char::::::acterized by a high probability of diabetes (53%) and CVD (52%) and a low probability of other diseases; therefore, we labeled this class “diabetes & CVDs.” This group also had the highest probability of CKD (10%). Class 2, comprising 0.6% of the patients, w::::::as char::::::acterized by a high probability of metabolic diseases (59%) and other diseases (75%); thus, we labeled this class “metabolic & other diseases.” the third class, which included 23.0% of the patients, w::::::as char::::::acterized by a moderate probability of hypertension (36%) and diabetes (40%), along with a low probability of other chronic diseases; therefore, we labeled this class “diabetes & hypertension”. The fourth class, with 72.7% of the patients, had a very low probability of all chronic conditions compared to other classes; we labeled this class “non-multimorbidity”. The probability of diabetes varied between 8% in class 4 to 53% in class 1. COPD, chronic liver disease, malignancy, immune system disease, chronic neurological diseases and asthma had low probabilities in all classes. The relative frequency of multimorbidity classes and mortality across age and gender groups is presented in Appendix 1.

Initially, an empty model with no predictors was fitted to examine the variation of death and ICU admissions across hospitals. The variance component and the standard error at the hospital level in the empty model were 0.35 and 0.10, respectively, resulting in an ICC of 9.6% for mortality. For ICU admissions, the variance component and the standard error were 0.99 and 0.25, respectively, yielding an ICC of 23.1%.
Then, the associations between predictors and outcomes (death and ICU admission) were examined using univariate analysis through two-level logistic regression (Table 3). In univariate analyses, the presence of some chronic diseases increased the odds ratio of admission to ICU (with the exception of liver, immune system, metabolic, and other diseases) and death from COVID-19 (except for liver, asthma, metabolic, and other diseases). Also, males, compared with females, as well as age groups 41-70 and >71 years, had higher odds of death (OR=1.27 for males; 3.08 and 9.49 for age groups 41-70 and >71, respectively) (Table 3).

The association between the multimorbidity classes of chronic diseases and ICU admission (model 1) and death (model 2), adjusted for gender and age groups, is shown in Table 4. Membership in class 1 (diabetes and CVD's) and class 3 (diabetes and hypertension) compared with class 4 (non-multimorbidity), was associated with higher odds of ICU admission (OR=2.12 for class 1 and 1.13 for class 3) and death (OR=2.66 for class 1 and 1.21 for class 3). Also, membership in class 2 compared with class 4 showed a higher odds ratio for experiencing ICU admission (OR=1.94, 95% CI, 1.06%, 3.55%), while this increase was not significant for death (OR=1.94, 95% CI, 0.47%, 3.55%). The ICC for models 1 and 2 showed that 23.3% and 6.5% of the total variance for ICU admission and death from COVID-19 were explained at the hospital level, respectively (Table 4).

Discussion
The results of the present study showed four patterns of multimorbidity in hospitalized COVID-19 patients, including diabetes and CVD (class 1, 3.7%), metabolic diseases and others (class 2, 0.6 %), diabetes and hypertension (class 3, 23.0%), and non-multimorbidity (class 4, 72.7%). The multimorbidity pattern with “diabetes and CVD's” in hospitalized COVID-19 patients had the highest chance of admission to ICU and mortality compared to other multimorbidity patterns. Also, our study showed that patients with comorbidities, particularly those with a history of COPD, diabetes, CVDs, CKD, neurological disease, hypertension, and malignancy, have a higher rate of ICU admission and death compared to other patients. The current study showed that multimorbidity is associated with serious adverse outcomes (ICU admission and death) even after adjusting for age groups and gender. Age was adjusted for because it is a strong confounder [20], as individuals who die from COVID-19 are often older and have more comorbidities [4, 10]. The present study is consistent with previous meta-analyses that indicate men have a higher mortality rate from COVID-19 [8].
Consistent with our study, Iaccarino et al. [20], in a study of 1,591 Italian patients diagnosed with COVID-19 who were admitted to 26 hospitals, found that among those who did not survive, there was a greater prevalence of hypertension, diabetes mellitus, COPD, CKD, coronary artery disease, and heart failure compared to the survivors. The results showed that multimorbidity from classes 1 and 3 increased the chances of COVID-19 mortality compared to patients with low morbidity (class 4). however, patients with predominantly metabolic and other diseases (class 2) did not show a significant difference in mortality compared to the low morbidity class. These results were similar for admission to ICU (as an outcome of severity). Nonetheless, patients with predominantly metabolic and other diseases had higher odds of admission to the ICU compared to patients with low morbidity.
Consistent with previous studies in Italy [20] and Denmark [21], our study showed an increase in ICU admissions and deaths related to multimorbidity. However, those studies used the Charlson comorbidity index (CCI) score to determine multimorbidity. In other words, the CCI was significantly higher in nonsurvivors compared to survivors.
Also, our results were consistent with a previous study in Italy [12] that showed an increased risk of mortality from COVID-19 for patients with cardiometabolic morbidity compared to those with no cardiometabolic conditions. In that study, cardiometabolic multimorbidity was defined as having at least two of three risk factors (diabetes, hypertension, and dyslipidemia).
According to COVID-19 pathogenicity analyses, most deaths caused by COVID-19 were due to cytokine storms caused by multiple organ dysfunction and acute respiratory distress syndrome (ARDS) [22]. A previous meta-analysis investigated the effect of co-infection on the onset of severe symptoms in patients with COVID-19 and showed that COPD, cerebrovascular disease, CVD, diabetes, and blood pressure, respectively, increased the risk of severe clinical symptoms [23]. Consistent with this meta-analysis, our study showed that the presence of comorbidity with malignancy, chronic neurological disease, CVD, CKD, immune system diseases, COPD, and diabetes increased the severity of COVID-19 and death rate in the univariate analysis. Various studies have shown that COPD is an essential indicator of severity and poor prognosis among COVID-19 patients [8]. In contrast, a previous study [23] indicated that CKD and malignancy did not affect the severity of clinical symptoms in COVID-19 patients. Among the comorbidities studied in various studies, the effect of malignancy on the prognosis of COVID-19 is a controversial issue. Liang et al. reported that people with malignancy had a higher risk of developing COVID-19 and a lower prognosis [24], a claim debated by other researchers [25, 26] due to the small sample size of Liang’s study and various confounding factors. However, the results of the meta-analyses conducted by Fang et al. [8] and the present study confirmed that the risk of death in COVID-19 patients increases with malignancy. Angiotensin-converting enzyme (ACE) is a significant regulator of blood pressure and serves as the binding site for the SARS-COV2 virus; for this reason, hypertension is one of the most common comorbidities associated with COVID-19 [27, 28], affecting disease severity and mortality. Iaccarino et al. [20] reported a higher frequency of ACE inhibitors, diuretics, and β-blocker usage among non-survivors compared to survivors.
Consistent with previous studies [5, 29], having more than one comorbidity compared to the absence of comorbidities increases the risk of death. Guan et al. [5] showed that in 31 Chinese provinces, the presence of two or more risk factors compared to the absence of risk factors increased the risk of requiring ventilation and death by 2.59 times, while the existence of one risk factor (hypertension or diabetes) raised the risk by 1.78 times. Agrawal et al. [29] found that in a cohort of patients hospitalized in Scotland due to COVID-19 during the first wave (between February 28, 2020 and September 22, 2020), the adjusted odds ratio for the existence of multimorbidity (≥2 comorbidities) compared to non-multimorbidity (≤1 comorbidity) was 1.49. It seems multimorbidity exacerbates pathological mechanisms in the three different phases of COVID-19 from the initial viral replication phase to inflammatory lung injury and post-acute sequelae—thereby reducing the patient’s tolerance to organ injury [30].
If CVD and malignancy are present in a person for a long time before the onset of COVID-19, they can increase the severity of disease and death from COVID-19. CVD, diabetes, and metabolic problems increase the risk of death from COVID-19 probably because they cause vascular endothelial injury, hemostatic system dysfunction, and pro-inflammatory state or chronic inflammation [31-33]. The simultaneous development of COVID-19 and heart disease, such as arrhythmia and atherosclerosis, increases the likelihood of thrombotic events, leading to an increased risk of fatal cerebral ischemia and acute stroke [22, 31].
Finally, our findings showed that the explained variations at the hospital level for mortality and ICU admission were about 10% and 23%, respectively. Therefore, the mortality associated with COVID-19 is less affected by hospital-level factors and more influenced by individual factors, such as comorbidity and demographic variables, such as age. This conclusion is confirmed by the fact that in model 2, the explained variation for COVID-19 mortality at the hospital level decreased after adjusting for age groups and gender (ICC decreased from 9.6% to 6.5%).
Conclusion
The classification based on multimorbidity proves to be a significant predictor for disease prognosis, aiding in treatment decisions and effective patient management. Importantly, the research demonstrates the persistent association between multimorbidity and severe adverse outcomes, such as ICU admission and mortality, even after adjusting for age and gender. This underscores the critical role of individual factors, particularly comorbidities, in determining COVID-19 outcomes. On the other hand, the variations in mortality and ICU admission at the hospital level indicate that individual-level factors exert a more substantial influence on COVID-19 mortality. This insight emphasizes the necessity of personalized approaches in managing COVID-19 cases. Also, preventive measures, including expanding protective recommendations and prioritizing vaccination for patients with multiple comorbidities, are essential. Building upon these findings, future research could delve deeper into understanding the specific mechanisms, through which certain comorbidities influence the severity of COVID-19. Investigating the molecular and physiological pathways involved could provide more targeted insights for treatment and intervention strategies.
Strengths and limitations
This study has notable strengths and limitations. This study utilized a robust methodological approach, employing LCA to determine multimorbidity patterns, accounting for response measurement errors and offering various goodness-of-fit indices for a more accurate portrayal of multimorbidity patterns. Additionally, the study leveraged extensive datasets from the national surveillance system of 42 hospitals in a province of Iran, marking the first investigation into the impact of multimorbidity patterns on COVID-19 patients in the country.
The study has limitations, including its focus on dichotomous outcomes for death, recovery, discharge, and ICU admission. Future research should explore the impact of multimorbidity patterns on years of life lost (YLL), length of hospitalization, and duration of ICU stay. The consideration of hospital-level factors and the potential information bias from self-reported morbidity data were acknowledged. Additionally, the study’s generalizability to outpatients, the examination of COVID-19 variants across different waves, and the regional nature of the findings in one province in northern Iran warrant attention in future investigations.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.1399.853). The collected data were anonymous and unidentifiable. Informed consent was obtained from all participations, and we confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Funding
The study was supported by Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization, study design and data collection: Mahmood Moosazadeh and Maysam Rezapour; Data analysis: Maysam Rezapour, Rahim Sharafkhani and Narges Khanjani; Writing the original draft: Rahim Sharafkhani; Review and editing: Maysam Rezapour and Narges Khanjani; Statistical analysis: Maysam Rezapour; Project administration, technical and material support: Mahmood Moosazadeh.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
In this research, the authors' colleague Bahram Tahmasebi was infected with COVID-19 and sadly passed away. The authors thank Bahram Tahmasebi for his contribution to this research.
References
The World Health Organization (WHO) announced the coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, as a pandemic in March 2020. It has spread rapidly in countries and territories and affected the lives and health of people worldwide. Although significant advances have been made in developing vaccines [1] and planning health measures [2] to prevent the disease and more effective therapies have been suggested, it is still important to identify potential factors affecting the severity, prognosis, and mortality of COVID-19. Additionally, effective triage for the treatment and management of patients is essential to allocate healthcare resources appropriately [3].
Epidemiological studies have shown that COVID-19 patients with chronic diseases experience higher hospitalization rates, increased disease severity, more intensive care unit (ICU) admissions, and ultimately higher mortality from COVID-19 [4-8]. Although some studies have examined the association between multimorbidity and mortality, several limitations exist in previous research. First, most prior studies have considered the comorbidity of chronic diseases separately [4-8]. This may lead to an inaccurate effect size, as many patients have multiple chronic diseases simultaneously. Therefore, considering multimorbidity (defined as the co-occurrence of at least two chronic conditions) in COVID-19 patients can provide better results for the identification of high-risk groups and informing treatment [9]. Second, while there is no consensus on the method used to measure multimorbidity, most previous studies have relied on counting the number of chronic comorbidities [5, 10-12], employing variable-centered approaches [13], or using specific cut points such as the Charlson comorbidity Index [14].
Latent class analysis (LCA), as a person-oriented grouping approach, is used to identify multimorbidity patterns [15]. In this method, patients are classified into latent clusters, which are homogeneous groups of individuals with regard to the multimorbidity of chronic diseases. In a previous study in Iran, five patterns of multimorbidity were found in the general population [15]. However, there is still no study on multimorbidity patterns among COVID-19 patients in Iran or other countries. Third, most previous studies have used relatively small sample sizes and have been based on single-center observations or have not accounted for hospital-level variations [4-6], when examining the associations between comorbidity (or multimorbidity) and outcomes, such as ICU admission and death. Hospital-level variations may be due to the hospital readiness dimensions of hospital readiness (such as incident management systems, coordination, information management, logistics, finance and administration, detection, diagnosis, isolation, case management and prevention and infection control) [16], the catchment area, the reputation of the hospitals and their physicians, tourist areas, and religious sites in the cities where the hospitals are located, among other factors. Finally, during the early phases of the COVID-19 pandemic, some general challenges faced by regions, including Mazandaran, could include issues related to healthcare infrastructure capacity due to the floating population caused by passengers, testing availability, public compliance with safety measures, and the overall management of the pandemic. Therefore, considering these challenges, this study investigated the patterns of multimorbidity among Iranian COVID-19 patients and their relation to ICU admission and death. The study opens avenues for further research, encouraging a deeper exploration of how to identify groups at risk for COVID-19 outcomes based on comorbidities and individual factors, as well as the development of more effective strategies for managing and treating patients.
Methods
Study population and design
In this retrospective cohort study, data were obtained from the surveillance system of the Medical Care Monitoring Center of the Ministry of Health and Medical Education (MOHME) of Iran, which has mandated all hospitals to register patients admitted with a diagnosis of confirmed or suspected COVID-19. The data of 13,960 COVID-19 patients from 42 hospitals in Mazandaran Province in northern Iran were collected according to the census between March 20, 2020 and July 20, 2021. These patients were hospitalized through three sources: direct visits to the hospital, referrals from outpatient centers (healthcare service centers) and referrals from other hospitals not designated for COVID-19 treatment. The inclusion criterion consisted of all positive COVID-19 patients admitted to the hospitals by RT-PCR during the specified time period, while the exclusion criterion included outpatients.
Measurement of chronic diseases
The included variables in this study were demographic characteristics (such as age and gender), the presence of chronic diseases as predictors, and ICU admission and/or death as outcomes. The chronic diseases included chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), diabetes, chronic kidney disease (CKD), chronic liver disease, malignancy, immune system diseases, chronic neurological disease, hypertension, asthma, metabolic diseases (hyper or hypo-thyroid and hyperlipidemia) and other diseases (rheumatoid arthritis and musculoskeletal diseases). These data were compiled based on the checklist prepared by the disease management center of the Ministry of Health and Medical Education, which was completed by each hospital or medical center upon admitting patients. The response options for each underlying chronic disease and for admission to the ICU and death were yes/no. Information about the morbidity of the patients was based on their medical files. Incomplete cases were addressed by reviewing the case files or through self-reporting from patients or their companions. If access to any of these sources was unavailable, the individual was excluded from the study.
Data analysis
Descriptive statistics were reported for all variables. LCA was used to extract multimorbidity patterns (grouping homogeneous patients in a class) based on the presence of different diseases. Then, we evaluated the relationship between each of these classes (or patterns) and the outcomes of COVID-19 (ICU admission and death) by adjusting age and gender via logistic regression analysis. The regression analyses were conducted using a multilevel method to account for the dependence of responses observed among patients belonging to the same hospital and to correct for its effects. LCA was conducted in an exploratory and iterative process with an increasing number of classes. The optimal number of classes was identified based on model fit indices in combination with empirical evidence and interpretability. Model fit was assessed using the Akaike information criterion (AIC), Bayesian information criterion (BIC), sample size adjusted bayesian information criterion (aBIC), the Lo–Mendell–Rubin likelihood ratio (LMR LR) test, and Vuong-Lo-Mendell-Rubin likelihood ratio (VLMR) Test. Smaller values of AIC, BIC, and aBIC indicated a better model fit [17]. The LMR is a likelihood ratio test that compares the estimated model to model to a model with one fewer class (k-1). If the LMR is not significant, the model fit will not improve by including an additional class in the model [18]. The VLMR, P are interpreted in the same manner as those of the LMR LR test. Entropy was used to assess the quality of member classification; a value closer to 1.0 indicates better classification [19].
Two-level logistic regression analysis (random intercept) was used to explore the association between each independent variable and the outcome variables (death and ICU admission) in univariate analysis. Given the clustering nature of the data, two-level (random intercept) logistic regression modeling was used, as patients were nested within hospitals. We then fitted multilevel logistic regression with the multimorbidity patterns obtained from LCA as independent variables and ICU admission (model 1) and death (model 2) as outcomes. In both models, age groups and gender were adjusted for confounders. Before running models 1 and 2, initially, a null or unconditional model was built to calculate the intra-class correlation coefficient (ICC) of hospitals. Data preparation and regression models were done in STATA software, version 16, and LCA was run in Mplus software, version 7.4.
Results
The descriptive statistics of all variables are shown in Table 1. Among the COVID-19 patients, 28.2% had one comorbidity, while 9.3% had two comorbidities, and 1.6% had three or more comorbidities. Additionally, 50.6% of patients were female and 20.1% were aged over 71. The most common comorbidities among COVID-19 patients were diabetes (20.6%) and hypertension (9.9%). The relative frequency of hospitalization for COVID-19 patients in the ICU and mortality from the disease was approximately 17% and 13%, respectively.

To extract multimorbidity patterns of chronic diseases, a series of latent class models were run, in which the number of classes ranged from 2 to 7. Figure 1 shows the goodness-of-fit indices for each model. The VLMR and LMR tests yielded significant P in all models; therefore, we could not use these indices as differentiators. The 4-class model was selected because the BIC and SSA-BIC values were smaller than other models, although the entropy was also lower than that of the others.

The probability of each chronic disease in the four classes (the conditional probabilities for each of the classes) is presented in Table 2. class 1, which constituted 3.7% of the patients, w::::::as char::::::acterized by a high probability of diabetes (53%) and CVD (52%) and a low probability of other diseases; therefore, we labeled this class “diabetes & CVDs.” This group also had the highest probability of CKD (10%). Class 2, comprising 0.6% of the patients, w::::::as char::::::acterized by a high probability of metabolic diseases (59%) and other diseases (75%); thus, we labeled this class “metabolic & other diseases.” the third class, which included 23.0% of the patients, w::::::as char::::::acterized by a moderate probability of hypertension (36%) and diabetes (40%), along with a low probability of other chronic diseases; therefore, we labeled this class “diabetes & hypertension”. The fourth class, with 72.7% of the patients, had a very low probability of all chronic conditions compared to other classes; we labeled this class “non-multimorbidity”. The probability of diabetes varied between 8% in class 4 to 53% in class 1. COPD, chronic liver disease, malignancy, immune system disease, chronic neurological diseases and asthma had low probabilities in all classes. The relative frequency of multimorbidity classes and mortality across age and gender groups is presented in Appendix 1.

Initially, an empty model with no predictors was fitted to examine the variation of death and ICU admissions across hospitals. The variance component and the standard error at the hospital level in the empty model were 0.35 and 0.10, respectively, resulting in an ICC of 9.6% for mortality. For ICU admissions, the variance component and the standard error were 0.99 and 0.25, respectively, yielding an ICC of 23.1%.
Then, the associations between predictors and outcomes (death and ICU admission) were examined using univariate analysis through two-level logistic regression (Table 3). In univariate analyses, the presence of some chronic diseases increased the odds ratio of admission to ICU (with the exception of liver, immune system, metabolic, and other diseases) and death from COVID-19 (except for liver, asthma, metabolic, and other diseases). Also, males, compared with females, as well as age groups 41-70 and >71 years, had higher odds of death (OR=1.27 for males; 3.08 and 9.49 for age groups 41-70 and >71, respectively) (Table 3).

The association between the multimorbidity classes of chronic diseases and ICU admission (model 1) and death (model 2), adjusted for gender and age groups, is shown in Table 4. Membership in class 1 (diabetes and CVD's) and class 3 (diabetes and hypertension) compared with class 4 (non-multimorbidity), was associated with higher odds of ICU admission (OR=2.12 for class 1 and 1.13 for class 3) and death (OR=2.66 for class 1 and 1.21 for class 3). Also, membership in class 2 compared with class 4 showed a higher odds ratio for experiencing ICU admission (OR=1.94, 95% CI, 1.06%, 3.55%), while this increase was not significant for death (OR=1.94, 95% CI, 0.47%, 3.55%). The ICC for models 1 and 2 showed that 23.3% and 6.5% of the total variance for ICU admission and death from COVID-19 were explained at the hospital level, respectively (Table 4).

Discussion
The results of the present study showed four patterns of multimorbidity in hospitalized COVID-19 patients, including diabetes and CVD (class 1, 3.7%), metabolic diseases and others (class 2, 0.6 %), diabetes and hypertension (class 3, 23.0%), and non-multimorbidity (class 4, 72.7%). The multimorbidity pattern with “diabetes and CVD's” in hospitalized COVID-19 patients had the highest chance of admission to ICU and mortality compared to other multimorbidity patterns. Also, our study showed that patients with comorbidities, particularly those with a history of COPD, diabetes, CVDs, CKD, neurological disease, hypertension, and malignancy, have a higher rate of ICU admission and death compared to other patients. The current study showed that multimorbidity is associated with serious adverse outcomes (ICU admission and death) even after adjusting for age groups and gender. Age was adjusted for because it is a strong confounder [20], as individuals who die from COVID-19 are often older and have more comorbidities [4, 10]. The present study is consistent with previous meta-analyses that indicate men have a higher mortality rate from COVID-19 [8].
Consistent with our study, Iaccarino et al. [20], in a study of 1,591 Italian patients diagnosed with COVID-19 who were admitted to 26 hospitals, found that among those who did not survive, there was a greater prevalence of hypertension, diabetes mellitus, COPD, CKD, coronary artery disease, and heart failure compared to the survivors. The results showed that multimorbidity from classes 1 and 3 increased the chances of COVID-19 mortality compared to patients with low morbidity (class 4). however, patients with predominantly metabolic and other diseases (class 2) did not show a significant difference in mortality compared to the low morbidity class. These results were similar for admission to ICU (as an outcome of severity). Nonetheless, patients with predominantly metabolic and other diseases had higher odds of admission to the ICU compared to patients with low morbidity.
Consistent with previous studies in Italy [20] and Denmark [21], our study showed an increase in ICU admissions and deaths related to multimorbidity. However, those studies used the Charlson comorbidity index (CCI) score to determine multimorbidity. In other words, the CCI was significantly higher in nonsurvivors compared to survivors.
Also, our results were consistent with a previous study in Italy [12] that showed an increased risk of mortality from COVID-19 for patients with cardiometabolic morbidity compared to those with no cardiometabolic conditions. In that study, cardiometabolic multimorbidity was defined as having at least two of three risk factors (diabetes, hypertension, and dyslipidemia).
According to COVID-19 pathogenicity analyses, most deaths caused by COVID-19 were due to cytokine storms caused by multiple organ dysfunction and acute respiratory distress syndrome (ARDS) [22]. A previous meta-analysis investigated the effect of co-infection on the onset of severe symptoms in patients with COVID-19 and showed that COPD, cerebrovascular disease, CVD, diabetes, and blood pressure, respectively, increased the risk of severe clinical symptoms [23]. Consistent with this meta-analysis, our study showed that the presence of comorbidity with malignancy, chronic neurological disease, CVD, CKD, immune system diseases, COPD, and diabetes increased the severity of COVID-19 and death rate in the univariate analysis. Various studies have shown that COPD is an essential indicator of severity and poor prognosis among COVID-19 patients [8]. In contrast, a previous study [23] indicated that CKD and malignancy did not affect the severity of clinical symptoms in COVID-19 patients. Among the comorbidities studied in various studies, the effect of malignancy on the prognosis of COVID-19 is a controversial issue. Liang et al. reported that people with malignancy had a higher risk of developing COVID-19 and a lower prognosis [24], a claim debated by other researchers [25, 26] due to the small sample size of Liang’s study and various confounding factors. However, the results of the meta-analyses conducted by Fang et al. [8] and the present study confirmed that the risk of death in COVID-19 patients increases with malignancy. Angiotensin-converting enzyme (ACE) is a significant regulator of blood pressure and serves as the binding site for the SARS-COV2 virus; for this reason, hypertension is one of the most common comorbidities associated with COVID-19 [27, 28], affecting disease severity and mortality. Iaccarino et al. [20] reported a higher frequency of ACE inhibitors, diuretics, and β-blocker usage among non-survivors compared to survivors.
Consistent with previous studies [5, 29], having more than one comorbidity compared to the absence of comorbidities increases the risk of death. Guan et al. [5] showed that in 31 Chinese provinces, the presence of two or more risk factors compared to the absence of risk factors increased the risk of requiring ventilation and death by 2.59 times, while the existence of one risk factor (hypertension or diabetes) raised the risk by 1.78 times. Agrawal et al. [29] found that in a cohort of patients hospitalized in Scotland due to COVID-19 during the first wave (between February 28, 2020 and September 22, 2020), the adjusted odds ratio for the existence of multimorbidity (≥2 comorbidities) compared to non-multimorbidity (≤1 comorbidity) was 1.49. It seems multimorbidity exacerbates pathological mechanisms in the three different phases of COVID-19 from the initial viral replication phase to inflammatory lung injury and post-acute sequelae—thereby reducing the patient’s tolerance to organ injury [30].
If CVD and malignancy are present in a person for a long time before the onset of COVID-19, they can increase the severity of disease and death from COVID-19. CVD, diabetes, and metabolic problems increase the risk of death from COVID-19 probably because they cause vascular endothelial injury, hemostatic system dysfunction, and pro-inflammatory state or chronic inflammation [31-33]. The simultaneous development of COVID-19 and heart disease, such as arrhythmia and atherosclerosis, increases the likelihood of thrombotic events, leading to an increased risk of fatal cerebral ischemia and acute stroke [22, 31].
Finally, our findings showed that the explained variations at the hospital level for mortality and ICU admission were about 10% and 23%, respectively. Therefore, the mortality associated with COVID-19 is less affected by hospital-level factors and more influenced by individual factors, such as comorbidity and demographic variables, such as age. This conclusion is confirmed by the fact that in model 2, the explained variation for COVID-19 mortality at the hospital level decreased after adjusting for age groups and gender (ICC decreased from 9.6% to 6.5%).
Conclusion
The classification based on multimorbidity proves to be a significant predictor for disease prognosis, aiding in treatment decisions and effective patient management. Importantly, the research demonstrates the persistent association between multimorbidity and severe adverse outcomes, such as ICU admission and mortality, even after adjusting for age and gender. This underscores the critical role of individual factors, particularly comorbidities, in determining COVID-19 outcomes. On the other hand, the variations in mortality and ICU admission at the hospital level indicate that individual-level factors exert a more substantial influence on COVID-19 mortality. This insight emphasizes the necessity of personalized approaches in managing COVID-19 cases. Also, preventive measures, including expanding protective recommendations and prioritizing vaccination for patients with multiple comorbidities, are essential. Building upon these findings, future research could delve deeper into understanding the specific mechanisms, through which certain comorbidities influence the severity of COVID-19. Investigating the molecular and physiological pathways involved could provide more targeted insights for treatment and intervention strategies.
Strengths and limitations
This study has notable strengths and limitations. This study utilized a robust methodological approach, employing LCA to determine multimorbidity patterns, accounting for response measurement errors and offering various goodness-of-fit indices for a more accurate portrayal of multimorbidity patterns. Additionally, the study leveraged extensive datasets from the national surveillance system of 42 hospitals in a province of Iran, marking the first investigation into the impact of multimorbidity patterns on COVID-19 patients in the country.
The study has limitations, including its focus on dichotomous outcomes for death, recovery, discharge, and ICU admission. Future research should explore the impact of multimorbidity patterns on years of life lost (YLL), length of hospitalization, and duration of ICU stay. The consideration of hospital-level factors and the potential information bias from self-reported morbidity data were acknowledged. Additionally, the study’s generalizability to outpatients, the examination of COVID-19 variants across different waves, and the regional nature of the findings in one province in northern Iran warrant attention in future investigations.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.1399.853). The collected data were anonymous and unidentifiable. Informed consent was obtained from all participations, and we confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Funding
The study was supported by Mazandaran University of Medical Sciences.
Authors' contributions
Conceptualization, study design and data collection: Mahmood Moosazadeh and Maysam Rezapour; Data analysis: Maysam Rezapour, Rahim Sharafkhani and Narges Khanjani; Writing the original draft: Rahim Sharafkhani; Review and editing: Maysam Rezapour and Narges Khanjani; Statistical analysis: Maysam Rezapour; Project administration, technical and material support: Mahmood Moosazadeh.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgments
In this research, the authors' colleague Bahram Tahmasebi was infected with COVID-19 and sadly passed away. The authors thank Bahram Tahmasebi for his contribution to this research.
References
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (delta) variant. New England Journal of Medicine. 2021; 385(7):585-94. [DOI:10.1056/NEJMoa2108891]
- Pormehdi Ganji Z, Ahmadzadeh A, Abhari S, Rezapour M. The associations between sources of vaccine news and the intention to change adherence to COVID-19 preventive health measures. Journal of Research and Health. 2023; 13(5):359-72. [DOI:10.32598/JRH.13.5.2163.1]
- Ernita M, Wibowo A. Tackling non-communicable diseases in Asia countries systematic review. KnE Life Sciences. 2019; 4(10):358-64. [DOI:10.18502/kls.v4i10.3739]
- Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its impact on patients with COVID-19. SN Comprehensive Clinical Medicine. 2020; 2(8):1069-76. [DOI:10.1007/s42399-020-00363-4]
- Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. European Respiratory Journal. 2020; 55(5):200054. [DOI:10.1183/13993003.00547-2020]
- Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. The Journals of Gerontology. 2020; 75(11):2224-30. [DOI:10.1093/gerona/glaa183]
- Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. International Journal of Infectious Diseases. 2020; 94:91-5. [DOI:10.1016/j.ijid.2020.03.017]
- Fang X, Li S, Yu H, Wang P, Zhang Y, Chen Z, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging. 2020; 12(13):12493-503. [DOI:10.18632/aging.103579]
- Prados-Torres A, Calderón-Larranaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: A systematic review. Journal of Clinical Epidemiology. 2014; 67(3):254-66. [DOI:10.1016/j.jclinepi.2013.09.021]
- Hanlon P, Chadwick F, Shah A, Wood R, Minton J, McCartney G, et al. COVID-19-exploring the implications of long-term condition type and extent of multimorbidity on years of life lost: A modelling study. Wellcome Open Research. 2021; 5:75. [DOI:10.12688/wellcomeopenres.15849.3] [PMID]
- Fernández-Niño JA, Guerra-Gómez JA, Idrovo AJ. Multimorbidity patterns among COVID-19 deaths: Proposal for the construction of etiological models. Revista Panamericana de Salud Pública. 2020; 44:e166-e. [DOI:10.26633/RPSP.2020.166]
- Maddaloni E, D’Onofrio L, Alessandri F, Mignogna C, Leto G, Pascarella G, et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: A multicentre retrospective study (CoViDiab II). Cardiovascular Diabetology. 2020; 19(1):164. [DOI:10.1186/s12933-020-01140-2]
- Roso-Llorach A, Violán C, Foguet-Boreu Q, Rodriguez-Blanco T, Pons-Vigués M, Pujol-Ribera E, et al. Comparative analysis of methods for identifying multimorbidity patterns: A study of ‘real-world’ data. BMJ Open. 2018; 8(3):e018986. [DOI:10.1136/bmjopen-2017-018986]
- Roffman C, Buchanan J, Allison G. Charlson comorbidities index. Journal of Physiotherapy. 2016; 62(3):171. [DOI:10.1016/j.jphys.2016.05.008]
- Khorrami Z, Rezapour M, Etemad K, Yarahmadi S, Khodakarim S, Mahdavi Hezaveh A, et al. The patterns of Non-communicable disease Multimorbidity in Iran: A Multilevel Analysis. Scientific Reports. 2020; 10(1):3034. [DOI:10.1038/s41598-020-59668-y]
- Hosseini SH, Saleh Tabari Y, Assadi T, Ghasemihamedani F, HabibiSaravi R. [Hospitals readiness in response to COVID-19 pandemic in Mazandaran province, Iran 2020 (Persian)]. Journal of Mazandaran University of Medical Sciences. 2021; 31(196):71-81. [Link]
- Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling: A Multidisciplinary Journal. 2007; 14(4):535-69. [DOI:10.1080/10705510701575396]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001; 88(3):767-78. [DOI:10.1093/biomet/88.3.767]
- Hatami H, Saeidi Kiasari MR, Rezapour M. The evaluation of diagnostic values of clinical symptoms for COVID-19 hospitalized patients in northern iran: The syndromic surveillance system data. Archives of Clinical Infectious Diseases. 2022; 17(1):e11746. [DOI:10.5812/archcid-117465]
- Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M, et al. Age and multimorbidity predict death among COVID-19 patients. Hypertension. 2020; 76(2):366-72. [DOI:10.1161/HYPERTENSIONAHA.120.15324]
- Christensen DM, Strange JE, Gislason G, Torp-Pedersen C, Gerds T, Fosbøl E, et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID-19 patients. Journal of General Internal Medicine. 2020; 35(9):2801-3. [DOI:10.1007/s11606-020-05991-z]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020; 8(4):420-2. [DOI:10.1016/S2213-2600(20)30076-X]
- Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging. 2020; 12(7):6049-57. [DOI:10.18632/aging.103000]
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. The Lancet Oncology. 2020; 21(3):335-7. [DOI:10.1016/S1470-2045(20)30096-6]
- Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. The Lancet Oncology. 2020; 21(4):e180. [DOI:10.1016/S1470-2045(20)30150-9]
- Wang H, Zhang L. Risk of COVID-19 for patients with cancer. The Lancet Oncology. 2020; 21(4):e181. [DOI:10.1016/S1470-2045(20)30149-2]
- Turner AJ, Hiscox JA, Hooper NM. ACE2: From vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences. 2004; 25(6):291-4. [DOI:10.1016/j.tips.2004.04.001]
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. Journal of Virology. 2020; 94(7):e00127-20. [DOI:10.1128/JVI.00127-20]
- Agrawal U, Azcoaga-Lorenzo A, Fagbamigbe AF, Vasileiou E, Henery P, Simpson CR, et al. Association between multimorbidity and mortality in a cohort of patients admitted to hospital with COVID-19 in Scotland. Journal of the Royal Society of Medicine. 2022; 115(1):22-30. [DOI:10.1177/01410768211051715]
- Russell CD, Lone NI, Baillie JK. Comorbidities, multimorbidity and COVID-19. Nature Medicine. 2023; 29(2):334-43. [DOI:10.1038/s41591-022-02156-9]
- Larson AS, Savastano L, Kadirvel R, Kallmes DF, Hassan AE, Brinjikji W. Coronavirus disease 2019 and the cerebrovascular-cardiovascular systems: What do we know so far? Journal of the American Heart Association. 2020; 9(13):e016793. [DOI:10.1161/JAHA.120.016793]
- Tadic M, Cuspidi C, Grassi G, Mancia G. COVID-19 and arterial hypertension: Hypothesis or evidence? The Journal of Clinical Hypertension. 2020; 22(7):1120-6. [DOI:10.1111/jch.13925]
- Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in Chinese patients with COVID-19: Systematic review and meta-analysis of risk factors. BMC Infectious Diseases. 2021; 21(200):200. [DOI:10.1186/s12879-021-05915-0]
Type of Study: Orginal Article |
Subject:
● Disease Control
Received: 2023/09/24 | Accepted: 2023/12/23 | Published: 2024/10/28
Received: 2023/09/24 | Accepted: 2023/12/23 | Published: 2024/10/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |