Volume 15, Issue 2 (March & April 2025)
J Research Health 2025, 15(2): 127-134 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Basaza R K, Kizito S, Kyasiimire E P. Delays in Diagnosis of Tuberculosis a Fishing Community in Uganda: A Cross-sectional Study. J Research Health 2025; 15 (2) :127-134
URL: http://jrh.gmu.ac.ir/article-1-2442-en.html
URL: http://jrh.gmu.ac.ir/article-1-2442-en.html
1- Department of Health Policy and Planning, School of Public Health, Gudie University Project, Kampala, Uganda. , rbasaza@gmail.com
2- Department of Public Health, Faculty of Public Health and Nursing, Uganda Christian University, Mukono, Uganda.
3- School of Public Health and Management, Clarke International University, Kampala, Uganda.
2- Department of Public Health, Faculty of Public Health and Nursing, Uganda Christian University, Mukono, Uganda.
3- School of Public Health and Management, Clarke International University, Kampala, Uganda.
Full-Text [PDF 573 kb]
(507 Downloads)
| Abstract (HTML) (3378 Views)
Full-Text: (704 Views)
Introduction
Worldwide tuberculosis (TB) is a major public health problem, especially in low and middle-income countries. In 2022 alone, Africa has 23% of all global cases of TB. Uganda ranks among the top 30 countries worldwide with a significant number of TB cases, resulting in a daily mortality rate of approximately 30 individuals. TB can manifest in two forms: Pulmonary TB, primarily affecting the lungs, and extra-pulmonary TB, which affects other areas of the body, including the abdomen. In an effort to eradicate TB, community action, case detection, and screening are being employed along with diligent follow-up on patients taking their medicine [1, 2]. Furthermore, in Uganda, 35% of TB patients are co-infected with HIV. The number of incident TB cases notified was 93,447 in 2022. This highlights the task ahead for Uganda to achieve the new ambitious global target of ending TB by the year 2035 [3].
Namayingo district in Uganda, the study area, has a high HIV prevalence of 22%, which is three times the national average prevalence of 6.2% [2], with a TB notification rate of 63% (entirely at primary healthcare facilities), which is less than the national notification rate and the 70% recommended by the World Health Organization (WHO). In this study, patient delay was defined as the time from the onset of the TB cardinal symptom to the first visit to a healthcare provider. Unacceptable patient delay was defined as a period of more than three weeks. Health facility delay was defined as the time taken from the first visit to a healthcare provider up to the time of TB diagnosis. Unacceptable health facility delay was defined as a delay of more than one week. Treatment delay was taken as the duration from when the diagnosis was made to when the patient was initiated on treatment, with any period beyond the same day considered a delay. Total delay was taken as the sum of patient delay, health facility delay, and treatment delay. Total delay is thus the time interval from the onset of symptoms until treatment initiation. This is a contributor to the poor indicators due to the prolonged delay from the onset of TB to the time of diagnosis and initiation of treatment [3, 4]. It may lead to disease progression, increased mortality, morbidity, and TB transmission in the community [5].
Uganda has a national TB and leprosy program headed by a program manager and several program officers who coordinate the following units: Prevention and health promotion, monitoring and evaluation, care and treatment services, laboratory services and policy, and regional TB and leprosy services. At the regional level, management and supervision of TB and leprosy services are performed by the regional TB and leprosy focal person (RTLP). There are currently 12 regions, which are aligned to the 12 MoH regional performance monitoring teams (RPMT) structure. At the district level, the district health officer (DHO) is responsible for the management of healthcare services delivery, including TB and Leprosy prevention and care. The DHO assigns a district health team member as the district TB and leprosy supervisor (DTLS), who is responsible for overseeing TB and Leprosy care and prevention services in the district. At the facility level, a health worker is assigned the responsibility of overseeing TB and leprosy care and prevention services. At both the district and health facility levels, TB and Leprosy care and prevention services are integrated into the general health services [6]. The relationship between HIV and TB, along with the increasing resistance to anti-TB drugs, has resulted in the need to study TB in high HIV prevalence areas. Delays in presentation, diagnosis, and treatment are some of the contributing factors to increased morbidity and mortality due to TB in different regions across the world. The purpose of this study was to identify factors associated with delays in diagnosis of pulmonary TB at primary care facilities in a high HIV fishing community of Namayingo district, Eastern Uganda.
Methods
This cross-sectional study was conducted among primary care facilities in a high HIV prevalence fishing community in Namayingo District, Eastern Uganda, between January 2022 and September 2023, using both qualitative and quantitative methods. A team of ten Research Assistants, who were health workers from the University and had expertise in data collection, supported the data collection exercise. The research assistants were trained to be very conversant with the study’s data collection techniques.
Sampling and sample size determination
The sample size was calculated using the Kish Leslie formula (Equation 1)
1. n=Z2pq/d2
where n is the sample size required for the study, Z is the z score at 95% confidence level (1.96) and P is the proportion of TB patients who had a diagnostic delay. In this study, the 90% used to estimate the sample size was adopted from a recent study in Mukono (Buregyeya et al. [5]) was used, where P=0.90 and d is the margin of error at a 95% level of significance, which was 0.05 (Equation 2).
2. n=[1.962×0.9×(1-0.9)]/0.052
n=138
Purposive sampling was employed in health facilities conducting TB testing. Clients diagnosed with TB (based on microscopy, GeneXpert, or clinical findings) and who had started on anti-TB drugs were selected from the records at the respective health facilities.
Inclusion and exclusion criteria
In this study, 140 clients qualified and accepted to participate based on the records accessed at the different health facilities. However, 14 participants failed to recall vital information required for this study and were excluded. As a result, we were left with only 126 adult patients (≥18 years) with pulmonary TB who had been receiving treatment for at least three months from both public and private not-for-profit primary care facilities. These respondents were identified retrospectively and included in the study while those who declined to participate were excluded from the study. Primary data were obtained from TB patients using interviewer-administered questionnaires. Secondary sources of data were also consulted to supplement the primary data, including health facility records. Pulmonary TB was diagnosed according to any of the following methods established in the Uganda TB treatment guidelines: 1) Detection by the Ziel-Neelsen method (microscopy) 2) Detection by the GeneXpert method and 3) The presence of clinical, epidemiological and radiological findings compatible with TB.
Qualitative data were obtained from purposively sampled respondents based on their roles and responsibilities regarding the management of TB in the selected health facilities in the district.
Data analysis
Quantitative data with a normal distribution by age and symptom presentation were entered into EpiData software, version 2.0.8.56 and exported to SPSS software, version 20 for analysis after cleaning. The collected data were analyzed and presented in frequency distribution tables as percentages using Microsoft Excel. Categorical comparisons were performed by the chi-square test. Multiple logistic regression analysis was used to evaluate factors associated with delay in univariate analysis. The regression analysis included confounding variables as covariates. Odds ratios and nominal 95% confidence intervals (CI) were presented. A two-sided P<0.05 was considered significant for all analyses.
Informed consent and anonymity
We obtained ethical approval to conduct this study from the Clarke International University Faculty Research Committee. To ensure confidentiality, respondents’ names were not recorded on the questionnaires; instead, codes were assigned to respondents for anonymity and confidentiality. In addition, the research team assured respondents that the sources of information provided throughout this study would remain undisclosed. Consequently, the research team sought informed consent from all respondents before data collection. Responses were ordinarily obtained without influence from colleagues, leaders, or data collectors. The respondents were informed that they could withdraw from the interview at any time if they wished to do so. None of the respondents in this study opted out before completing the study.
Results
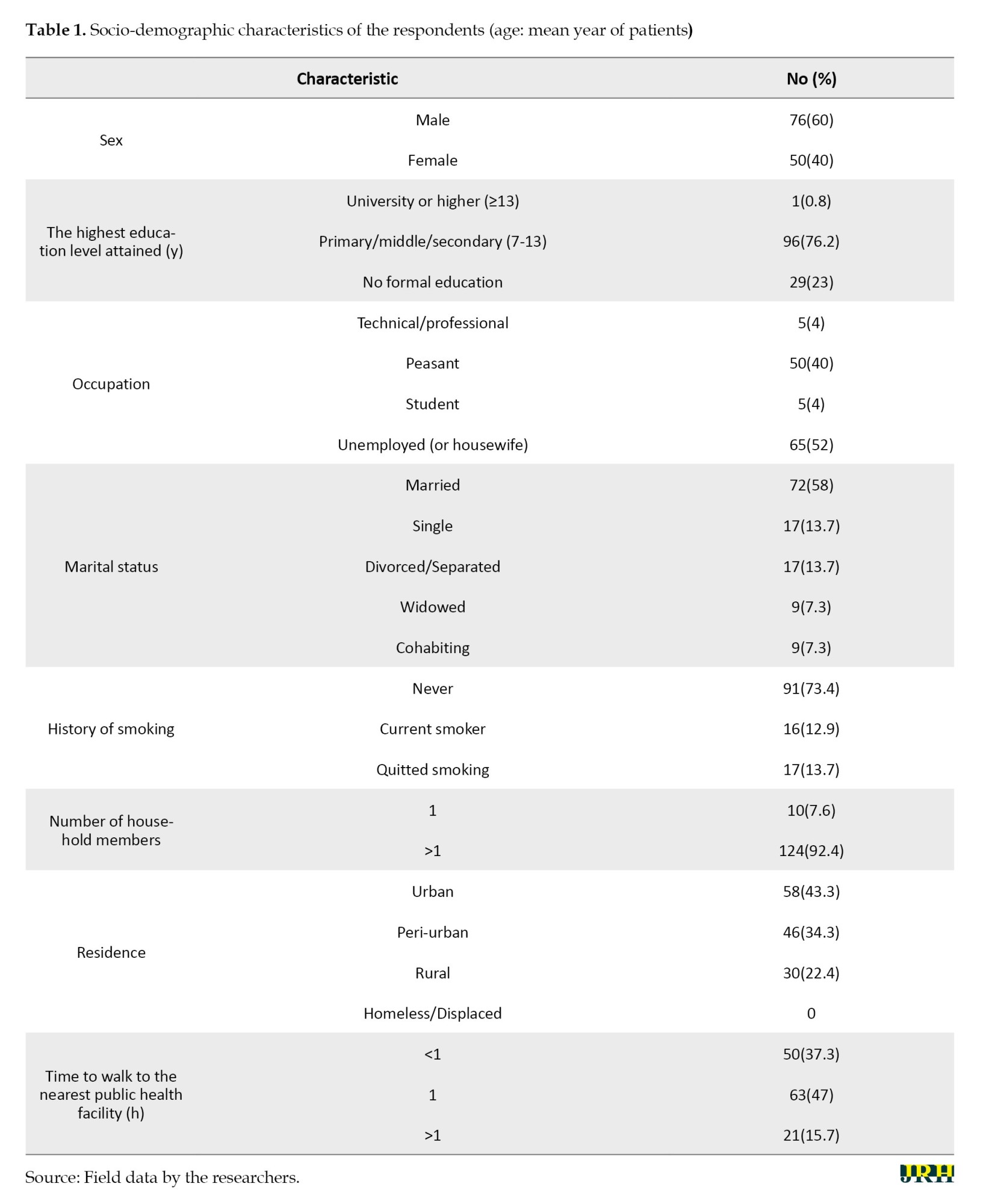
One hundred twenty-six respondents met the inclusion criteria and were considered in the analysis. The socio-demographic characteristics of the study population are shown in Table 1.
Total diagnostic delay was prevalent in 69% of the respondents, with a median of eight weeks (zero to six weeks). Patient delay was the greatest contributor to total delay; it was prevalent in 64% of clients and had a median of six weeks (zero to ten weeks), whereas health facility delay had a median of only two weeks (zero to zero weeks) and was prevalent in 50% of clients. The distribution of delays is presented in Table 2.

Chi-square tests revealed no individual factors associated with delays: Gender (P<0.152), occupation (P<0.480), marital status (P<0.801) and history of smoking (P<0.166). However, health facility delay was significantly associated with how well a health facility was equipped (P<0.0002) and the actions taken by the healthcare provider at the point of the first consultation by the patient (P<0.018).
Logistical regression analysis was conducted to estimate the odds ratios (ORs) for the risk of diagnostic delay. Other variables considered included gender, occupation, marital status, primary health care (PHC), level of equipment, quality of health services, stigma, education, knowledge about TB, and actions taken by the health workers. The same variables were included in the multivariate analysis. Gender (OR=0.234, 95% CI, 0.041%, 0.962%; P<0.037) and patient knowledge about TB (OR=6.804 95% CI, 1.547%, 28.821%; P<0.006) were significantly associated with delay. The predictors of unacceptable delay are presented in Tables 3 and 4.
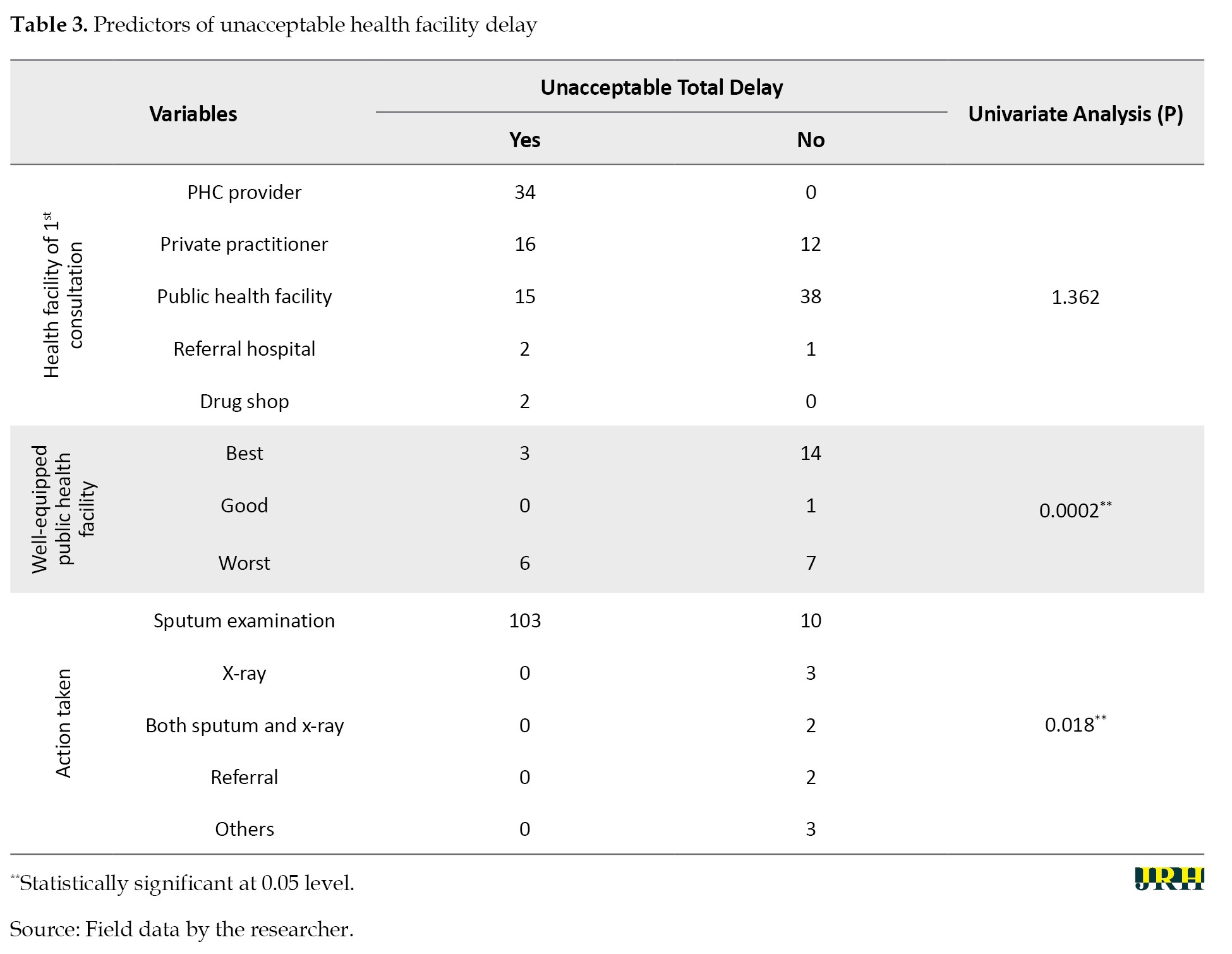
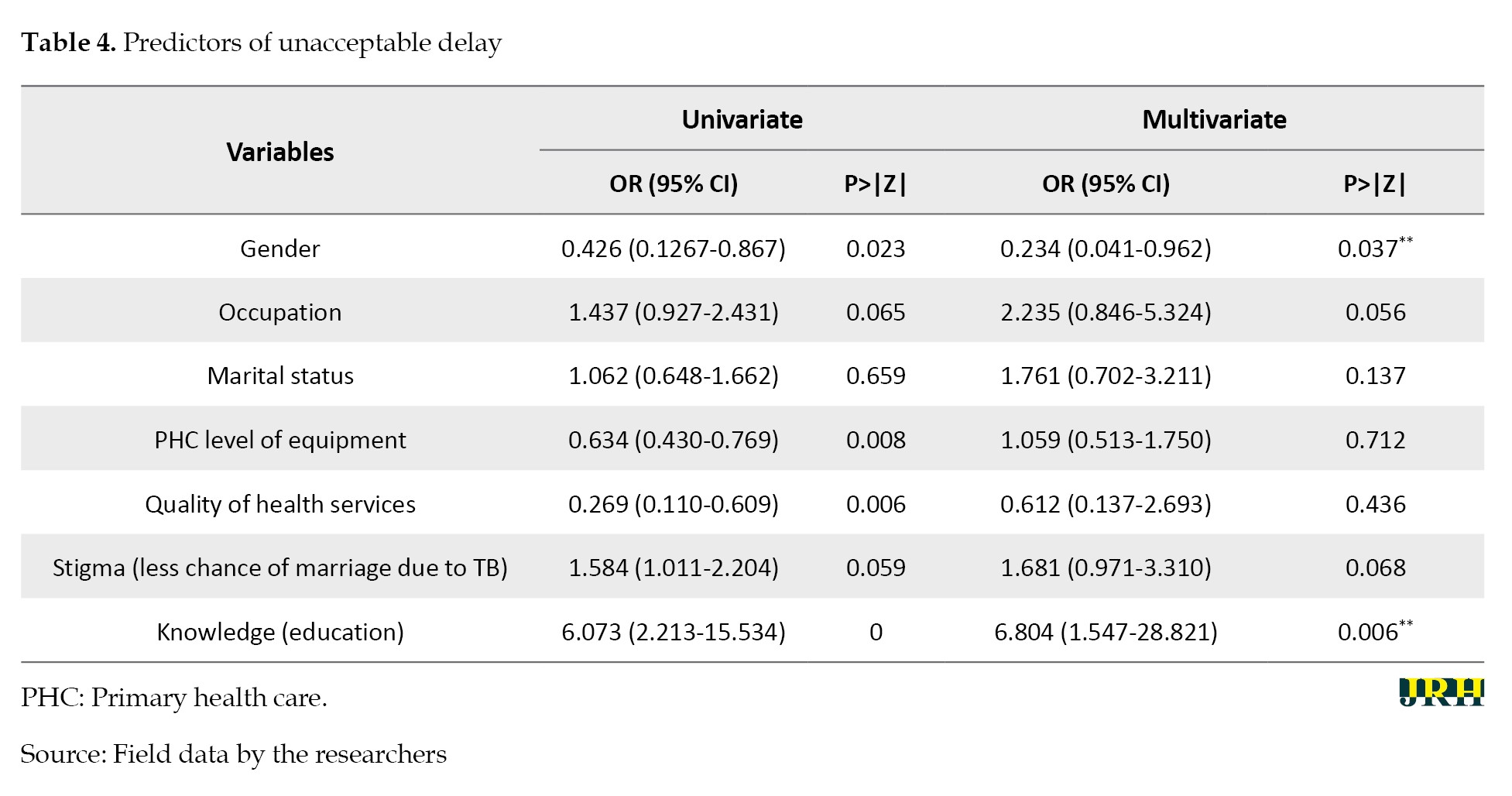
Health facility testing delay was significantly associated with how well a health facility was equipped for TB diagnostics, as indicated by the chi-square test (P<0.0002). The equipping of health facilities was primarily from public care providers, and this was significant in the delay, with an OR of 0.634 at a (95% CI, 0.430%, 0.769%) (P<0.008). Other factors associated with delay included actions taken by the healthcare provider during the first consultation. More delay (P<0.018) was associated with instances where only a sputum examination was conducted (103/113). In instances where both sputum and x-ray examinations were performed, no delay was reported.
Health facility factors associated with TB diagnostic delay
The testing delay was 46.5%, with a median delay of zero days.
Discussion
This study found that the median total TB diagnostic delay was eight weeks, with a prevalence of 69.4%, and that patient delay was the leading contributor to total delay, with a median of 6 weeks and a prevalence of 64%. This finding is consistent with a study by Oola, which found that patient delay contributed more than health facility delay [4]. This can be attributed to the fact that Oola’s study was conducted in a similar rural setting as this study. However, this finding differs from a similar study conducted in Wakiso and Mukono districts in Uganda, where health services delay was the biggest contributor to total TB diagnostic delay [5, 7]. The improvements in diagnostic capacities of many laboratories and the introduction of more sensitive tests, like the GeneXpert technology, could have contributed to a reduction in health system delay; thus leaving patient delay as the leading contributor to total delay.
The median total delay of eight weeks was lower than that of 16 weeks in the study done in Mukono in 2014, lower than the 12 weeks and lower than the 12 weeks found in a study by Buregyeya et al. in Kampala [4, 5]. The differences in the study settings could have led to these varying findings. The studies in Kampala, Mukono, and Wakiso were conducted in and around the capital city, which is more urbanized and has relatively more health facilities, many of which are not accredited to treat TB patients, resulting in longer delays. In contrast, this study site was a rural fishing community with fewer facilities, the majority of which are accredited, leading to a shorter delay. Other reasons for the reduced diagnostic delay (eight weeks) were attributed to the recent introduction of more sensitive GeneXpert machines and the functionalization of the hub system at health facilities, which reduced the turnaround time for TB diagnosis and, consequently, health system delay.
Gender: This was found to be a significant determinant of delays, with being male associated with a longer diagnostic delay compared to being female. This finding is similar to a study conducted in Mukono [5]; the similarity may be due to the fact that both studies were conducted in rural settings and in primary healthcare facilities. However, these findings differ from many other similar studies that found females to have longer diagnostic delays than males. A similar study in Iran found that females had longer diagnostic delays [8]. Also, the findings of a similar study in Bangladesh revealed that females had longer diagnostic delays compared to males [9]. The difference in findings is attributed to the fact that in rural fishing communities, where the current study was conducted, males are often involved in fishing activities, which are characterized by routine movement from one island to another and overnight duties, leaving them with barely any time to access the few available health facilities, unlike the findings of other studies.
Patients’ knowledge about TB disease: This was found to be a significant predictor of patient delay in this study. This finding is similar to a study that found limited knowledge about TB disease was associated with patient diagnostic delay [10]. Another study also found that limited knowledge about the mode of transmission of TB contributed significantly to patient diagnostic delay [11]. These studies and our findings indicate that people with limited knowledge about TB are unlikely to take the initiative to seek medical care, highlighting the need to educate the public about TB in order to reduce the many weeks patients take before seeking appropriate care.
The limitations of the study included recall bias among the study participants and inadequate documentation at the health facilities. The recall bias was supplemented with available records, such as TB treatment cards and case notes, for further insights; where records were not available, information was obtained from the client’s next of kin. The study was carried out in primary healthcare facilities that were both public and private not-for-profit, but not in any private for-profit facilities, which limits the generalizability of our findings.
Conclusion
The factors associated with TB diagnostic delay were patient knowledge about TB and gender. Reducing these delays requires the Ministry of Health and its partners to increase community awareness about TB, improve diagnostic awareness among health workers, and ensure the timely supply of diagnostic equipment and reagents to health facilities. It is anticipated that the study findings could assist health policymakers and practitioners in devising suitable interventions that may lead to increased TB case detection, improved treatment, and reduced transmission of infection in the community, thereby achieving effective TB control. Further studies should focus on explaining the factors that influence the health-seeking behaviors of clients in high HIV prevalence fishing communities and elucidate the role of primary healthcare providers, including community health workers, in combating TB. Additionally, the role of traditional healers in the delayed diagnosis of TB in a high HIV prevalence community could be explored.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Clarke International University, Kampala, Uganda (Code: CLARKE-2020-9). All study respondents provided informed consent prior to enrollment.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their gratitude to the study participants, research assistants, staff and health facility in-charges and Namayingo district health officials. The authors are also grateful to the university authorities for the ethical clearance and support of this study.
References
Worldwide tuberculosis (TB) is a major public health problem, especially in low and middle-income countries. In 2022 alone, Africa has 23% of all global cases of TB. Uganda ranks among the top 30 countries worldwide with a significant number of TB cases, resulting in a daily mortality rate of approximately 30 individuals. TB can manifest in two forms: Pulmonary TB, primarily affecting the lungs, and extra-pulmonary TB, which affects other areas of the body, including the abdomen. In an effort to eradicate TB, community action, case detection, and screening are being employed along with diligent follow-up on patients taking their medicine [1, 2]. Furthermore, in Uganda, 35% of TB patients are co-infected with HIV. The number of incident TB cases notified was 93,447 in 2022. This highlights the task ahead for Uganda to achieve the new ambitious global target of ending TB by the year 2035 [3].
Namayingo district in Uganda, the study area, has a high HIV prevalence of 22%, which is three times the national average prevalence of 6.2% [2], with a TB notification rate of 63% (entirely at primary healthcare facilities), which is less than the national notification rate and the 70% recommended by the World Health Organization (WHO). In this study, patient delay was defined as the time from the onset of the TB cardinal symptom to the first visit to a healthcare provider. Unacceptable patient delay was defined as a period of more than three weeks. Health facility delay was defined as the time taken from the first visit to a healthcare provider up to the time of TB diagnosis. Unacceptable health facility delay was defined as a delay of more than one week. Treatment delay was taken as the duration from when the diagnosis was made to when the patient was initiated on treatment, with any period beyond the same day considered a delay. Total delay was taken as the sum of patient delay, health facility delay, and treatment delay. Total delay is thus the time interval from the onset of symptoms until treatment initiation. This is a contributor to the poor indicators due to the prolonged delay from the onset of TB to the time of diagnosis and initiation of treatment [3, 4]. It may lead to disease progression, increased mortality, morbidity, and TB transmission in the community [5].
Uganda has a national TB and leprosy program headed by a program manager and several program officers who coordinate the following units: Prevention and health promotion, monitoring and evaluation, care and treatment services, laboratory services and policy, and regional TB and leprosy services. At the regional level, management and supervision of TB and leprosy services are performed by the regional TB and leprosy focal person (RTLP). There are currently 12 regions, which are aligned to the 12 MoH regional performance monitoring teams (RPMT) structure. At the district level, the district health officer (DHO) is responsible for the management of healthcare services delivery, including TB and Leprosy prevention and care. The DHO assigns a district health team member as the district TB and leprosy supervisor (DTLS), who is responsible for overseeing TB and Leprosy care and prevention services in the district. At the facility level, a health worker is assigned the responsibility of overseeing TB and leprosy care and prevention services. At both the district and health facility levels, TB and Leprosy care and prevention services are integrated into the general health services [6]. The relationship between HIV and TB, along with the increasing resistance to anti-TB drugs, has resulted in the need to study TB in high HIV prevalence areas. Delays in presentation, diagnosis, and treatment are some of the contributing factors to increased morbidity and mortality due to TB in different regions across the world. The purpose of this study was to identify factors associated with delays in diagnosis of pulmonary TB at primary care facilities in a high HIV fishing community of Namayingo district, Eastern Uganda.
Methods
This cross-sectional study was conducted among primary care facilities in a high HIV prevalence fishing community in Namayingo District, Eastern Uganda, between January 2022 and September 2023, using both qualitative and quantitative methods. A team of ten Research Assistants, who were health workers from the University and had expertise in data collection, supported the data collection exercise. The research assistants were trained to be very conversant with the study’s data collection techniques.
Sampling and sample size determination
The sample size was calculated using the Kish Leslie formula (Equation 1)
1. n=Z2pq/d2
where n is the sample size required for the study, Z is the z score at 95% confidence level (1.96) and P is the proportion of TB patients who had a diagnostic delay. In this study, the 90% used to estimate the sample size was adopted from a recent study in Mukono (Buregyeya et al. [5]) was used, where P=0.90 and d is the margin of error at a 95% level of significance, which was 0.05 (Equation 2).
2. n=[1.962×0.9×(1-0.9)]/0.052
n=138
Purposive sampling was employed in health facilities conducting TB testing. Clients diagnosed with TB (based on microscopy, GeneXpert, or clinical findings) and who had started on anti-TB drugs were selected from the records at the respective health facilities.
Inclusion and exclusion criteria
In this study, 140 clients qualified and accepted to participate based on the records accessed at the different health facilities. However, 14 participants failed to recall vital information required for this study and were excluded. As a result, we were left with only 126 adult patients (≥18 years) with pulmonary TB who had been receiving treatment for at least three months from both public and private not-for-profit primary care facilities. These respondents were identified retrospectively and included in the study while those who declined to participate were excluded from the study. Primary data were obtained from TB patients using interviewer-administered questionnaires. Secondary sources of data were also consulted to supplement the primary data, including health facility records. Pulmonary TB was diagnosed according to any of the following methods established in the Uganda TB treatment guidelines: 1) Detection by the Ziel-Neelsen method (microscopy) 2) Detection by the GeneXpert method and 3) The presence of clinical, epidemiological and radiological findings compatible with TB.
Qualitative data were obtained from purposively sampled respondents based on their roles and responsibilities regarding the management of TB in the selected health facilities in the district.
Data analysis
Quantitative data with a normal distribution by age and symptom presentation were entered into EpiData software, version 2.0.8.56 and exported to SPSS software, version 20 for analysis after cleaning. The collected data were analyzed and presented in frequency distribution tables as percentages using Microsoft Excel. Categorical comparisons were performed by the chi-square test. Multiple logistic regression analysis was used to evaluate factors associated with delay in univariate analysis. The regression analysis included confounding variables as covariates. Odds ratios and nominal 95% confidence intervals (CI) were presented. A two-sided P<0.05 was considered significant for all analyses.
Informed consent and anonymity
We obtained ethical approval to conduct this study from the Clarke International University Faculty Research Committee. To ensure confidentiality, respondents’ names were not recorded on the questionnaires; instead, codes were assigned to respondents for anonymity and confidentiality. In addition, the research team assured respondents that the sources of information provided throughout this study would remain undisclosed. Consequently, the research team sought informed consent from all respondents before data collection. Responses were ordinarily obtained without influence from colleagues, leaders, or data collectors. The respondents were informed that they could withdraw from the interview at any time if they wished to do so. None of the respondents in this study opted out before completing the study.
Results
One hundred twenty-six respondents met the inclusion criteria and were considered in the analysis. The socio-demographic characteristics of the study population are shown in Table 1.

Total diagnostic delay was prevalent in 69% of the respondents, with a median of eight weeks (zero to six weeks). Patient delay was the greatest contributor to total delay; it was prevalent in 64% of clients and had a median of six weeks (zero to ten weeks), whereas health facility delay had a median of only two weeks (zero to zero weeks) and was prevalent in 50% of clients. The distribution of delays is presented in Table 2.

Chi-square tests revealed no individual factors associated with delays: Gender (P<0.152), occupation (P<0.480), marital status (P<0.801) and history of smoking (P<0.166). However, health facility delay was significantly associated with how well a health facility was equipped (P<0.0002) and the actions taken by the healthcare provider at the point of the first consultation by the patient (P<0.018).
Logistical regression analysis was conducted to estimate the odds ratios (ORs) for the risk of diagnostic delay. Other variables considered included gender, occupation, marital status, primary health care (PHC), level of equipment, quality of health services, stigma, education, knowledge about TB, and actions taken by the health workers. The same variables were included in the multivariate analysis. Gender (OR=0.234, 95% CI, 0.041%, 0.962%; P<0.037) and patient knowledge about TB (OR=6.804 95% CI, 1.547%, 28.821%; P<0.006) were significantly associated with delay. The predictors of unacceptable delay are presented in Tables 3 and 4.


Health facility testing delay was significantly associated with how well a health facility was equipped for TB diagnostics, as indicated by the chi-square test (P<0.0002). The equipping of health facilities was primarily from public care providers, and this was significant in the delay, with an OR of 0.634 at a (95% CI, 0.430%, 0.769%) (P<0.008). Other factors associated with delay included actions taken by the healthcare provider during the first consultation. More delay (P<0.018) was associated with instances where only a sputum examination was conducted (103/113). In instances where both sputum and x-ray examinations were performed, no delay was reported.
Health facility factors associated with TB diagnostic delay
The testing delay was 46.5%, with a median delay of zero days.
Discussion
This study found that the median total TB diagnostic delay was eight weeks, with a prevalence of 69.4%, and that patient delay was the leading contributor to total delay, with a median of 6 weeks and a prevalence of 64%. This finding is consistent with a study by Oola, which found that patient delay contributed more than health facility delay [4]. This can be attributed to the fact that Oola’s study was conducted in a similar rural setting as this study. However, this finding differs from a similar study conducted in Wakiso and Mukono districts in Uganda, where health services delay was the biggest contributor to total TB diagnostic delay [5, 7]. The improvements in diagnostic capacities of many laboratories and the introduction of more sensitive tests, like the GeneXpert technology, could have contributed to a reduction in health system delay; thus leaving patient delay as the leading contributor to total delay.
The median total delay of eight weeks was lower than that of 16 weeks in the study done in Mukono in 2014, lower than the 12 weeks and lower than the 12 weeks found in a study by Buregyeya et al. in Kampala [4, 5]. The differences in the study settings could have led to these varying findings. The studies in Kampala, Mukono, and Wakiso were conducted in and around the capital city, which is more urbanized and has relatively more health facilities, many of which are not accredited to treat TB patients, resulting in longer delays. In contrast, this study site was a rural fishing community with fewer facilities, the majority of which are accredited, leading to a shorter delay. Other reasons for the reduced diagnostic delay (eight weeks) were attributed to the recent introduction of more sensitive GeneXpert machines and the functionalization of the hub system at health facilities, which reduced the turnaround time for TB diagnosis and, consequently, health system delay.
Gender: This was found to be a significant determinant of delays, with being male associated with a longer diagnostic delay compared to being female. This finding is similar to a study conducted in Mukono [5]; the similarity may be due to the fact that both studies were conducted in rural settings and in primary healthcare facilities. However, these findings differ from many other similar studies that found females to have longer diagnostic delays than males. A similar study in Iran found that females had longer diagnostic delays [8]. Also, the findings of a similar study in Bangladesh revealed that females had longer diagnostic delays compared to males [9]. The difference in findings is attributed to the fact that in rural fishing communities, where the current study was conducted, males are often involved in fishing activities, which are characterized by routine movement from one island to another and overnight duties, leaving them with barely any time to access the few available health facilities, unlike the findings of other studies.
Patients’ knowledge about TB disease: This was found to be a significant predictor of patient delay in this study. This finding is similar to a study that found limited knowledge about TB disease was associated with patient diagnostic delay [10]. Another study also found that limited knowledge about the mode of transmission of TB contributed significantly to patient diagnostic delay [11]. These studies and our findings indicate that people with limited knowledge about TB are unlikely to take the initiative to seek medical care, highlighting the need to educate the public about TB in order to reduce the many weeks patients take before seeking appropriate care.
The limitations of the study included recall bias among the study participants and inadequate documentation at the health facilities. The recall bias was supplemented with available records, such as TB treatment cards and case notes, for further insights; where records were not available, information was obtained from the client’s next of kin. The study was carried out in primary healthcare facilities that were both public and private not-for-profit, but not in any private for-profit facilities, which limits the generalizability of our findings.
Conclusion
The factors associated with TB diagnostic delay were patient knowledge about TB and gender. Reducing these delays requires the Ministry of Health and its partners to increase community awareness about TB, improve diagnostic awareness among health workers, and ensure the timely supply of diagnostic equipment and reagents to health facilities. It is anticipated that the study findings could assist health policymakers and practitioners in devising suitable interventions that may lead to increased TB case detection, improved treatment, and reduced transmission of infection in the community, thereby achieving effective TB control. Further studies should focus on explaining the factors that influence the health-seeking behaviors of clients in high HIV prevalence fishing communities and elucidate the role of primary healthcare providers, including community health workers, in combating TB. Additionally, the role of traditional healers in the delayed diagnosis of TB in a high HIV prevalence community could be explored.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Clarke International University, Kampala, Uganda (Code: CLARKE-2020-9). All study respondents provided informed consent prior to enrollment.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors extend their gratitude to the study participants, research assistants, staff and health facility in-charges and Namayingo district health officials. The authors are also grateful to the university authorities for the ethical clearance and support of this study.
References
- World Health Organization (WHO). Global tuberculosis report. 1.1 TB incidence [Internet]. 2023 [Updated 2024 September 20]. Available from: [Link]
- Ministry of Health. The path towards a TB-free Uganda by 2030 [Internet]. 2023 [Updated 2024 September 20]. Available from: [Link]
- Ministry of Health. Annual report. Abuja: National TB & Leprosy Control Division; 2023. [Link]
- Oola J. Factors influencing delayed Diagnosis of Tuberculosis in Mukono District, Uganda [Internet]. 2001 [Updated 10 Sept. 2023]. Available from: [Link]
- Buregyeya E, Criel B, Nuwaha F, Colebunders R. Delays in diagnosis and treatment of pulmonary tuberculosis in Wakiso and Mukono districts, Uganda. BMC Public Health. 2014; 14:586. [DOI:10.1186/1471-2458-14-586] [PMID]
- Ministry of Health. Uganda HIV/AIDS country progress report July 2016-June 2017. Kampala: Uganda AIDS Commission; 2017. [Link]
- Kiwuwa MS, Charles K, Harriet MK. Patient and health service delay in pulmonary tuberculosis patients attending a referral hospital: A cross-sectional study. BMC Public Health. 2005; 5:122. [DOI:10.1186/1471-2458-5-122] [PMID]
- Alavi SM, Bakhtiyariniya P, Albagi A. Factors associated with delay in diagnosis and treatment of pulmonary tuberculosis. Jundishapur Journal of Microbiology. 2015; 8(3):e19238. [DOI:10.5812/jjm.19238]
- Karim F, Islam MA, Chowdhury AM, Johansson E, Diwan VK. Gender differences in delays in diagnosis and treatment of tuberculosis. Health Policy and Planning. 2007; 22(5):329-34. [DOI:10.1093/heapol/czm026] [PMID]
- Hamza A, Demissie M, Gare S, Teshome G. Delay in tuberculosis diagnosis among tuberculosis patients at the three hospitals: Asella, robe and abomsa of arsi zone, oromia regional state, March, 2015. Open Access Library Journal. 2015; 2(12):1-3. [DOI:10.4236/oalib.1101947]
- Kola O, Bruce K, Stavia T, Nikki D, Jeanne C, Winters M, et al. Quality of tuberculosis services assessment in Uganda: Measure evaluation. Chapel Hill: Measure Evaluation; 2020. [Link]
Type of Study: Orginal Article |
Subject:
● Health Systems
Received: 2023/10/8 | Accepted: 2024/10/15 | Published: 2025/03/2
Received: 2023/10/8 | Accepted: 2024/10/15 | Published: 2025/03/2
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |