Volume 14, Issue 4 (Jul & Aug 2024)
J Research Health 2024, 14(4): 313-328 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Acharya P, Mir S A, Nayak B. Aedes Mosquito Dynamics: Unravelling Behavior, Genetics, and Arbovirus Risks in India. J Research Health 2024; 14 (4) :313-328
URL: http://jrh.gmu.ac.ir/article-1-2472-en.html
URL: http://jrh.gmu.ac.ir/article-1-2472-en.html
1- Department of Biotechnology, Sambalpur University, Sambalpur, India.
2- Department of Lifesciences, Sambalpur University, Sambalpur, India.
3- Department of Lifesciences, Sambalpur University, Sambalpur, India. ,binatanayak@suniv.ac.in
2- Department of Lifesciences, Sambalpur University, Sambalpur, India.
3- Department of Lifesciences, Sambalpur University, Sambalpur, India. ,
Full-Text [PDF 2731 kb]
(1473 Downloads)
| Abstract (HTML) (3841 Views)
Full-Text: (2145 Views)
Introduction
Dengue is a predominant arboviral disease transmitted by Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus mosquitoes, pose a significant global threat, contributing to an annual burden of 100–400 million new infections. Approximately 3.83 billion people are at risk worldwide [1, 2]. These vectors also transmit other impactful diseases, such as chikungunya, yellow fever, and Zika infections [3]. The spectrum of dengue infections encompasses mild fever to severe forms, like dengue hemorrhagic fever and dengue shock syndrome, driven by four distinct serotypes (DENV–1, DENV–2, DENV–3, and DENV–4) capable of co-infecting humans [3, 4]. Dengue clinical manifestations include fever, rash, eye pain, arthralgias, myalgias, and hemorrhage [3].
The quantification of the global burden of dengue is challenging due to widespread under-reporting, especially in areas with weaker healthcare systems [5, 6]. Dengue is widespread, affecting over 120 countries, especially in the Americas, Southeast Asia, and the Western Pacific [5, 6]. The global spread of dengue began in the 1980s, reaching various regions, such as the Mediterranean basin, the Americas, and Africa [7]. Brazil has the highest number of reported cases in the Americas, with 4.1 million, while Africa, particularly Burkina Faso, is experiencing a significant increase in the number of cases [7, 8]. Dengue is also reported in Eastern Mediterranean countries, with the emergence of local cases and the expansion of Ae. albopictus in Europe [9]. In the Western Pacific Region, which includes Australia, China, and the Philippines, there are over 500000 reported cases [7]. About 70% of the disease burden is concentrated in Asian countries [2, 5, 6]. South-East Asian nations, like India, Indonesia, Myanmar, Sri Lanka, and Thailand rank among the top 30 countries globally with the highest dengue prevalence, as per the Centers for Disease Control and Prevention 2024 [4, 10].
India’s contribution to global dengue infections is estimated at 34 out of 96 million, significantly contrasting with the reported 12 484 cases to the World Health Organization (WHO), revealing a substantial underreporting gap [2]. However, in India, there is a lack of clear understanding of the distribution of dengue vectors, although their presence has been confirmed in specific regions through local surveillance efforts [11-13]. Major regions in India, are affected by one of the four arboviral infections [14]. Furthermore, a study on the impact of climate change on mosquito abundance in India suggests an increased risk in parts of the Thar Desert and the Upper Himalayas [15]. Despite lower reported dengue cases, there is a high prevalence observed in northeast India and the western coastline, emphasizing the need for heightened monitoring [11]. Ae. aegypti dominates in the Deccan plateau and arid regions of Gujarat and Rajasthan, while Ae. albopictus prevails in the eastern coastline, aligning with reported dengue incidence in Gujarat, Maharashtra, Punjab, and Karnataka [11, 15].
Ae. aegypti and Ae. albopictus showcase adaptability shaped by their evolutionary history and genetic diversity [9, 16]. Ae. aegypti has two subspecies, namely Ae. aegypti aegypti and the ancestral Ae. aegypti formosus, originating from Africa, with the former adapting to urban settings and becoming a primary vector [16]. Ae. albopictus, from Southeast Asia, displays versatility in human-altered environments, spreading worldwide [8]. Behaviorally, Ae. aegypti prefers indoor resting and daytime human feeding, mainly in urban areas and artificial water containers [3, 4]. Conversely, Ae. albopictus, an opportunistic feeder with outdoor resting tendencies, also exhibits anthropophagic behavior [1, 4]. Factors influencing their distribution include urbanization, climate elements (temperature, rainfall, and humidity), and human mobility [1, 4, 6, 11, 17]. Human movement contributes to lower genetic differentiation [15], while changing precipitation patterns impact larval habitats, affecting vector population dynamics [16]. The intricate interplay of these factors contributes to the rapid expansion of vectors and their prevalence worldwide [1, 4, 6, 8, 9, 11, 17].
Despite the increasing number of dengue cases, there is no specific treatment or vaccine available [2, 7, 10, 14]. In India, dengue control relies on different practices, such as indoor spraying, fogging, and personal protection promotion, yet strong evidence of the effectiveness of these interventions is lacking [18, 19]. This underscores the urgent need for tailored control measures. Conventional surveillance often overlooks factors, like seasonal larval abundance fluctuations and non-residential areas within cities, where Aedes mosquitoes thrive [20]. The impact of urbanization, social behavior, and climate change on Aedes mosquitoes in India are not well understood. Recent studies highlight challenges in controlling Ae. aegypti and Ae. albopictus in Western Africa and Thailand due to insufficient research on their behavior [1, 4]. Recognizing the global impact of Aedes-borne diseases, our study addresses knowledge gaps in Aedes mosquito behavior, genetic diversity, and arbovirus transmission in India [1, 4]. To enhance surveillance and guide control efforts, this study pinpoints research gaps in Aedes mosquito behavior in different regions of residential areas and non-residential areas [11, 17, 20].
Methods
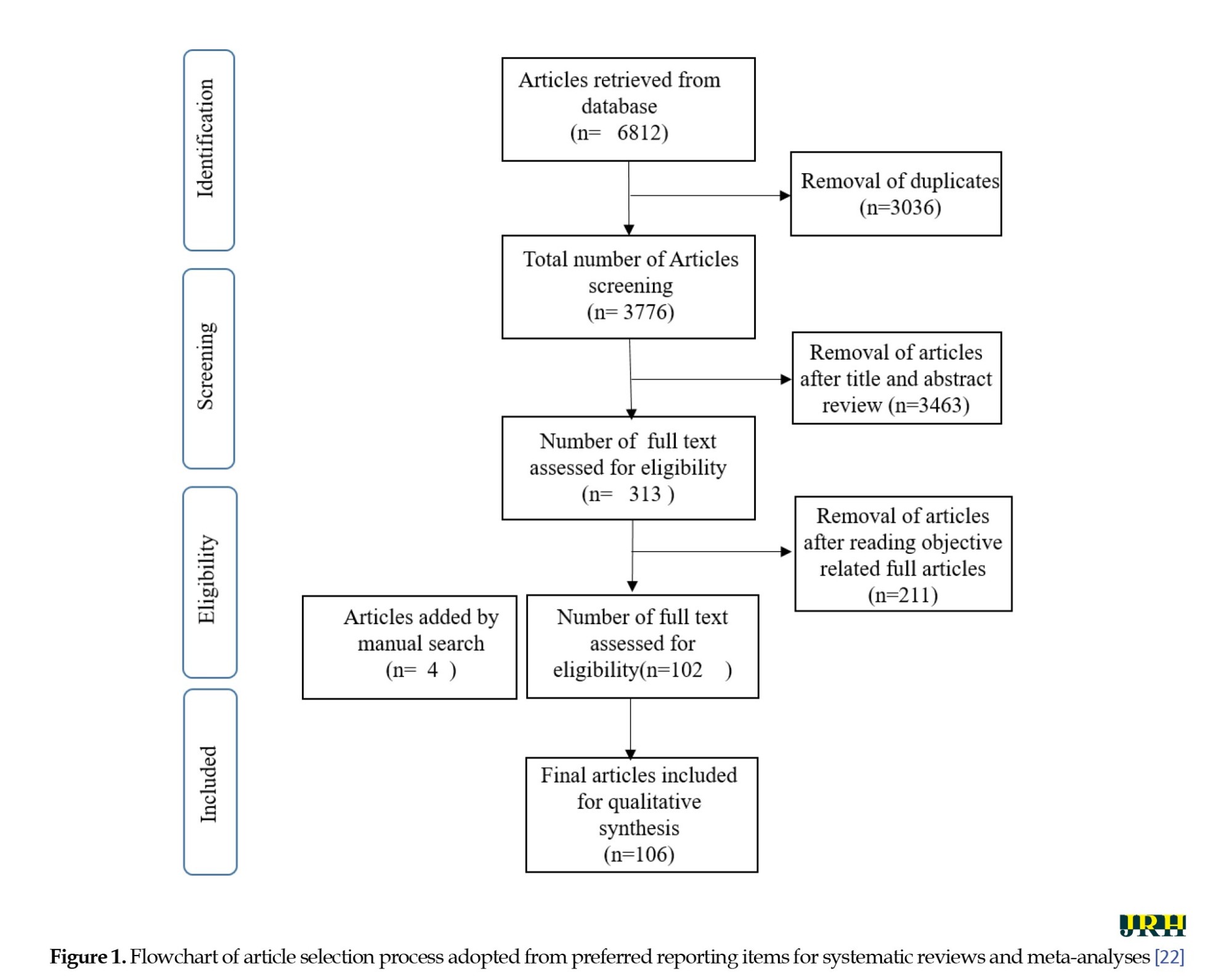
This study employed a scoping review approach by following the methodology from [21]. The article selection process involved five steps as follows: Defining the research question, conducting a thorough search for relevant studies, filtering and selecting pertinent articles, synthesizing and interpreting collected data, finally establishing the analytical framework, and reporting the results. Searches were conducted in Google Scholar, Web of Science, Scopus, and PubMed from October to November 2023, focusing on articles published between 2013 and November 2023. We have subdivided the second step of our search strategy into three sub-step processes: 1) An initial search of published studies in Google Scholar, Scopus, PubMed, and Web of Science databases; 2) Utilization of keywords and terms to search additional databases, including Science Direct, relevant government ministry portals (e.g. National Center for Vector Borne Diseases Control (NCVBDC)) and organizations (e.g. WHO and centers for disease control and prevention) by using Google search engine and manual search; 3) Exploration of the reference lists of identified studies for supplementary sources. Appropriate keywords were used to search literature in English language only and articles in other languages were removed. Whenever feasible, we used the preferred reporting items for systematic reviews and meta-analysis criteria [22] for performing and writing the results (Figure 1).
The keywords used to search literature are as follows:
● “Ae. aegypti” AND “dengue” AND “India”. “Aedes albopictus” AND “dengue” AND “India”
● “Ae. aegypti” AND “chikungunya” AND “India”. “Ae. albopictus” AND “chikungunya” AND “India”
● “Ae. aegypti” AND “Zika” AND “India”. “Ae. albopictus” AND “Zika” AND “India”
● “Ae. aegypti” AND “breeding” or “breeding habitat” AND “India”. “Ae. albopictus” AND “breeding” AND “India”
● “Ae. aegypti” AND “feeding preference” or “feeding behavior” or “blood-feeding” AND “India”. “Ae. albopictus” AND “feeding preference” or “feeding behavior” or “blood-feeding” AND “India”
● “Ae. aegypti” AND “genetic diversity” or “genetic structure” or “genetic variability” or AND “India”. “Ae. albopictus” AND “genetic diversity” or “genetic structure” or “genetic variability” or AND “India”
● “Aedes mosquitoes” AND “virus transmission” or “vertical transmission” or “transovarial transmission” AND “India”
The inclusion criteria for this review article were limited to full articles in the English language, concentrating on the status of Aedes borne diseases in India, Aedes mosquito behavior, including feeding behavior, breeding habitats, genetic diversity, arbovirus transmission dynamics in mosquitoes, and patterns of transmission in hosts. The review considered the influence of external factors, like ecological elements (including rainfall, temperature, and humidity) and social factors of urbanization, on mosquito adaptation to changing environments and their increased expansion. Data collection involved research on Ae. aegypti and Ae. albopictus across diverse states in India. The quality of the articles was checked and stored manually. Meanwhile, the exclusion criteria targeted articles that did not focus on the behavior and genetic diversity of Aedes mosquitoes in the Indian context, articles related to mosquitoes other than Aedes and the removal of duplicated or commonly addressed articles. The screening criteria for selecting the objective-related articles included having relevance to the study subject.
Results
Following an initial search and review of titles and abstracts, we identified a total of 102 articles from the online search and an additional 4 articles through manual searching. The details of these articles are outlined in Table 1. To align with the study objective, the extracted data from these articles were categorized into specific themes, including “Aedes mosquito biology and arboviral disease in India,” with sub-categories, such as “arboviral disease in India,” “virus transmission dynamics,” “feeding behavior, host preference, and biting pattern,” “breeding habitat,” and “genetic diversity.”

Arboviral disease in India
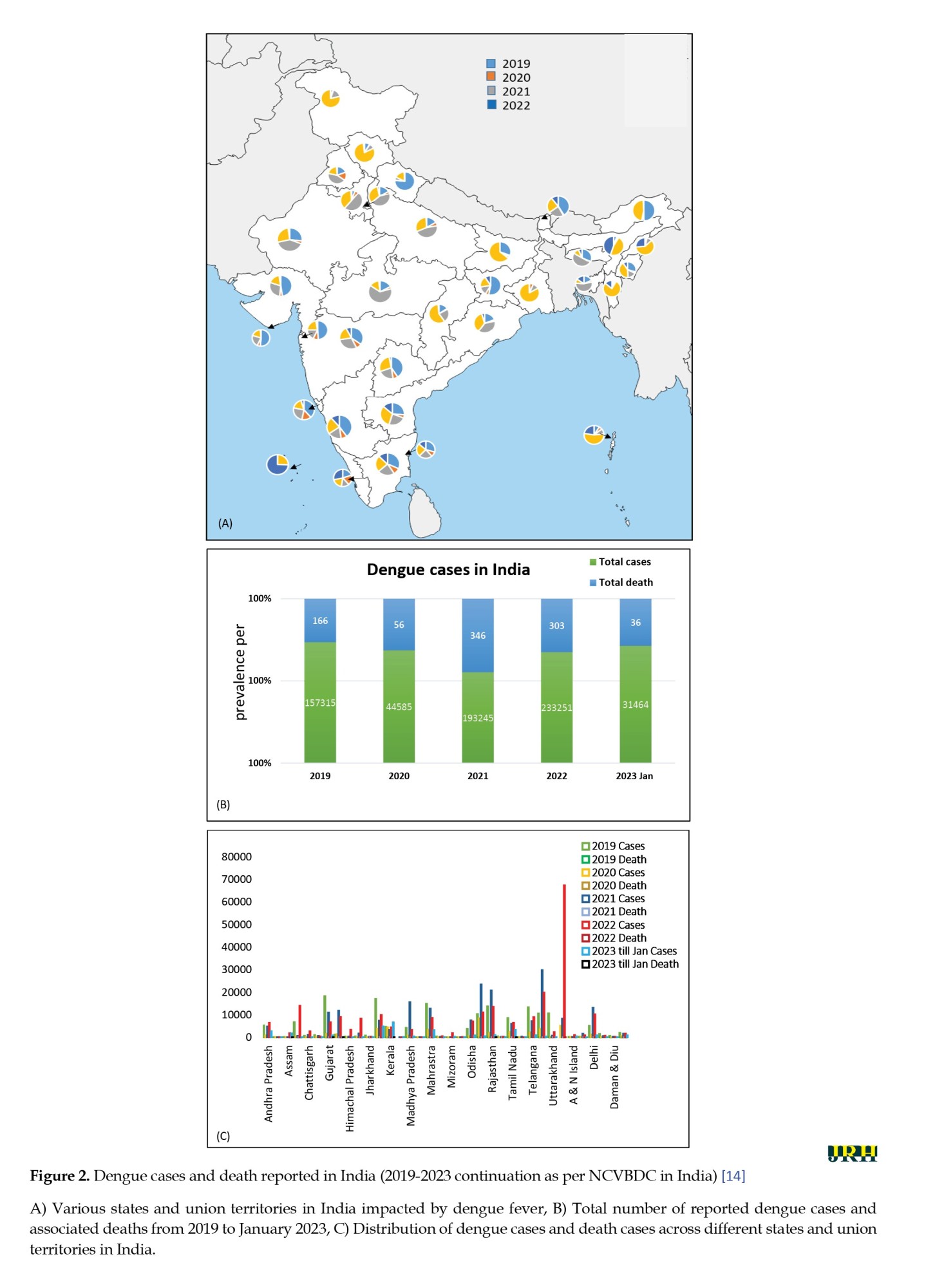
In the past two decades, India has witnessed a substantial geographic spread of dengue, experiencing repeated outbreaks and a staggering 11-fold surge in cases [23]. The global dengue outbreak in 2016, coupled with Delhi’s severe outbreak in 2015, underscored the widespread impact of the virus [23]. Dengue’s presence in India dates back to 1950, with the isolation of the first Dengue serotype, DENV-1, in Vellore, Tamilnadu, in 1956, followed by a major outbreak in Calcutta in 1963 [23]. Since the 1960s, outbreaks have been reported across all states and :union: territories, except Ladakh and Lakshadweep [23]. The emergence of dengue hemorrhagic fever intensified outbreaks, particularly in Delhi in 1996 and North India in 2003, highlighting the virus’s variability [24, 25]. According to the NCVBDC, data as of January 2023 (Figures 2A, 2B, and 2C) depict the distribution of reported dengue cases and deaths in India. Subsequent reports in September 2023 indicate a sustained impact of dengue fever, with persistently high cases in states like Kerala, Karnataka, Maharashtra, Odisha, Uttar Pradesh, Assam, Delhi, Telangana, Rajasthan, Tamil Nadu, West Bengal, and Punjab [14, 26].
A nationally representative survey in India unveiled age-specific dengue seroprevalence, estimating an overall prevalence of 48.7%. Variations were observed, ranging from 28.3% in children aged 5–8 years to 56.2% in individuals aged 18–45 years [27]. Geographical differences were evident, with the highest seroprevalence in southern regions (76.9%) and urban areas (70.9%), underscoring the impact of urbanization on dengue incidence [27]. Furthermore, genomic analyses reveal the co-circulation of all four dengue serotypes in different regions of India, with DENV-4 emerging as a dominant serotype in South India [28]. Diagnosis involves detecting NS1 antigen and IgM dengue antibody. Vaccines like TAK-003 are undergoing trials, facing challenges due to diverse antigens and genotype variations [29].
Chikungunya fever in India, caused by Chikungunya virus (CHIKV), is endemic in 24 states and 6 :union: territories [30]. The virus reemerged in 2005, causing a significant outbreak across states, with CHIKV’s adaptability to Ae. albopictus mosquitoes contributing to its rapid spread [31]. Molecular diagnostics, particularly reverse transcription polymerase chain reaction, play a crucial role in diagnosing acute-phase CHIKV infections [30]. Additionally, the Zika virus (ZIKV), with a complex evolutionary history, surfaced in Gujarat and Maharashtra in 1954, reappearing notably in Ahmedabad in 2016, Jaipur in 2018, and Madhya Pradesh in 2021 [32-34]. The continuous emergence of these viruses underscores the importance of robust surveillance, prompt diagnosis, and effective prevention strategies tailored to region-specific strains [33, 34].
Virus transmission dynamics
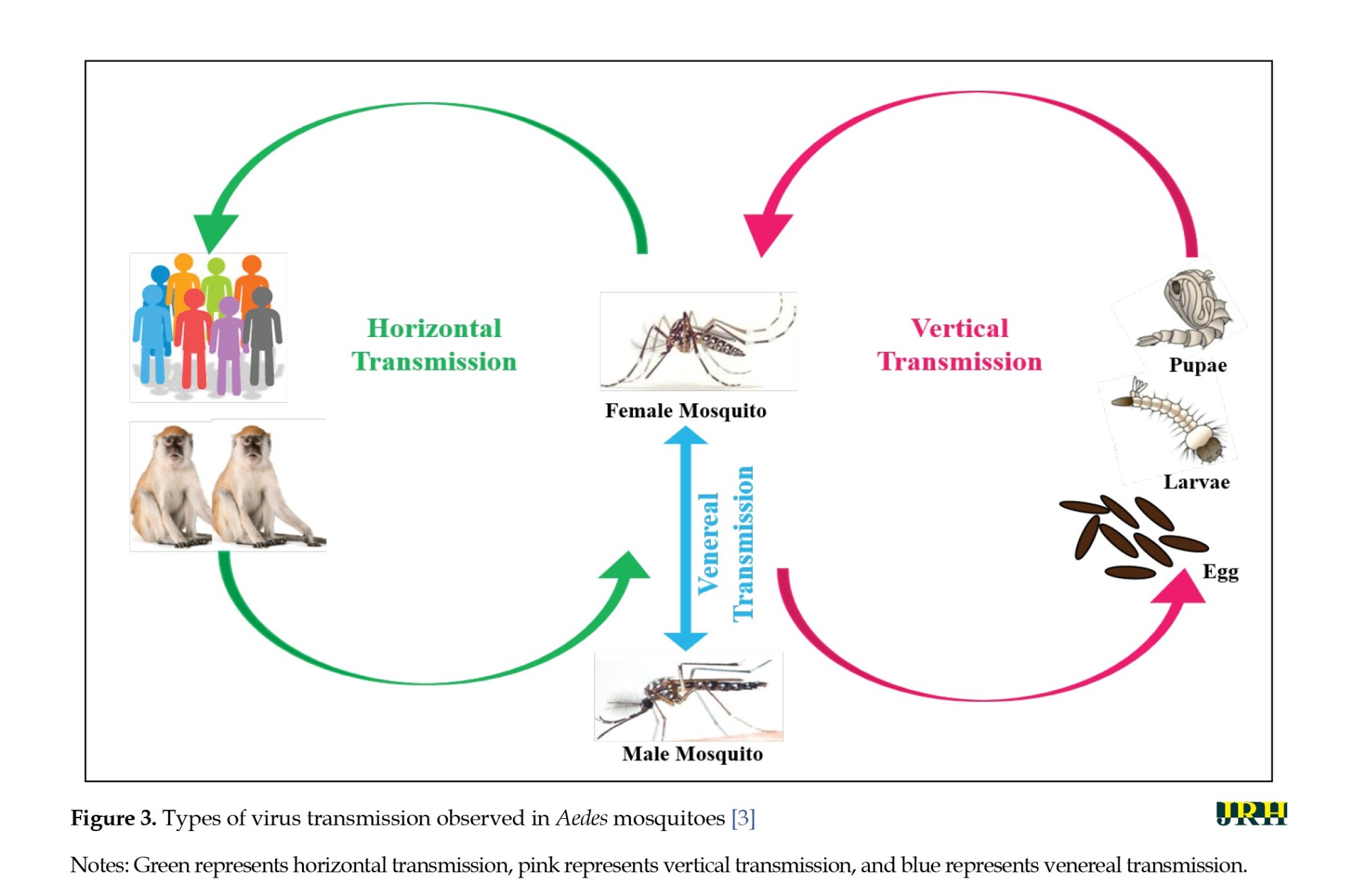
Arboviruses, transmitted primarily by mosquitoes, utilize horizontal and vertical transmission mechanisms. Horizontal transmission occurs during a blood meal, while vertical transmission involves passing the virus from an infected female mosquito to her offspring through eggs (Figure 3) [35, 36]. Understanding these mechanisms is crucial for studying dengue fever epidemiology. Global studies, including in India, confirm natural vertical transmission of dengue virus in Aedes mosquitoes [37]. Indian studies since 2007 show natural vertical transmission of dengue serotypes, varying globally, with South America and Asia contributing significantly to cases [35]. Africa lacks sufficient research on natural vertical transmission [38].
In India, studies in various states have shed light on the intricate dynamics of arbovirus transmission, particularly in Chennai, emphasizing the role of vertical transmission in maintaining dengue viruses during inter-epidemic periods [39]. Kerala reports natural vertical transmission in Ae. albopictus [40]. Rajasthan investigates dengue-3 virus persistence in successive Ae. aegypti generations, emphasizing re-emergence [41]. Uttar Pradesh reveals natural vertical transmission of dengue virus seen in male Aedes mosquitoes [42]. Delhi’s 2013 outbreak shows Ae. aegypti infection rates contribute to transmission in low and medium-income areas [43]. In Jodhpur, Bhopal, Aedes mosquitoes tested positive for dengue antigen via vertical transmission [44, 45]. Odisha faced rising dengue cases, prompting studies on DENV transmission dynamics [46].
Furthermore, vector surveillance is crucial, as seen in central India’s year-round xenomonitoring [47]. Nationwide surveillance initiated by the Indian Council of Medical Research from 2016 to 2019 across 14 states contributed to our understanding of ZIKV and DENV prevalence in Aedes mosquitoes [48]. Thiruvananthapuram’s intensive vector surveillance during a ZIKV outbreak detected high Aedes mosquito infestation [33].
Feeding behavior, host preference, and biting pattern
Hematophagy, essential for arthropod vector life cycles, underscores the frequent blood feeding of disease vectors like Ae. aegypti and Ae. albopictus, occurring every 2 to 4 days, is crucial for pathogen acquisition and transmission [49]. These mosquitoes exhibit a strong preference for human blood, influenced by environmental factors and host availability [50, 51]. In Asia, where Aedes mosquitoes are prevalent, they serve as competent vectors for various infectious diseases [2]. The body size of these mosquitoes, influenced by temperature and nutrition, impacts their ecology and multiple blood-feeding activities [52]. A study in the South Andaman district found that both Ae. aegypti and Ae. albopictus predominantly feeds on humans, with Ae. albopictus exhibiting a broader host range [53]. In Dehradun City, variations in mosquito abundance based on different habitats and seasons were observed, with Aedes mosquitoes primarily feed on humans, followed by bovines and pigs [54]. Understanding biting rhythms is also crucial, as evidenced by a study in Sivaganga district identifying three main rhythmic patterns among various mosquito species [55]. Additionally, the innovative approach of using attractive toxic sugar baits in India has shown efficacy in reducing mosquito populations by exploiting the sugar-feeding behavior of both male and female mosquitoes [56, 57].
Breeding habitat
India’s diverse landscapes and climates create a complex scenario for Aedes mosquito breeding, key vectors for diseases like dengue [58]. Aedes mosquitoes are adaptable to various habitats, from clean water receptacles to polluted collections like stagnant drain water, tanks, coolers, and containers [59]. Ae. aegypti favors indoor breeding, while Ae. albopictus prefers outdoor areas, like car tires and garbage dumps [59]. Visual and olfactory cues guide their oviposition site selection, considering both chemical and physical factors in the water [58]. Extensive studies across India emphasize Aedes mosquitoes’ adaptability to diverse breeding habitats, with larval indices, like house index, container index, and breteau index, serving as crucial tools for assessing dengue epidemiological risk [60]. In Delhi and Tamil Nadu, significant Aedes breeding dynamics occur in key containers like overhead tanks, enhancing cost-effective and sustainable control efforts [61, 62].
Kerala, susceptible to arboviral diseases, reveals unconventional breeding habitats in crops and brackish water areas [63, 64]. Northern Kerala studies link physicochemical parameters with adult emergence, providing insights into the relationship between water quality and mosquito presence [65]. The adaptability of Ae. albopictus to diverse environments, including discarded automobile tires and water-holding plastic containers have been observed in the Andaman Islands [66]. Various regions, such as Kanyakumari, Tripura, Thrissur, Bengaluru, Odisha, Bihar, Dehradun, and West Bengal identify specific containers, like plastic containers, tires, and water coolers as high-risk areas for Aedes breeding [20, 60, 67-77]. Entomological surveillance international airports underscore varying degrees of receptivity to Aedes vector breeding, emphasizing the importance of consistent monitoring and timely source reduction efforts [76].
Genetic diversity
In India, rising dengue cases necessitate a multidisciplinary approach, integrating morphological, molecular, and distributional data [78]. Our data analysis focuses on two key aspects as follows: First, understanding the origin and evolution of dengue serotypes, and exploring how they adapt to changing environments is one aspect. Secondly, we delve into the genetic variation within Indian Aedes mosquito populations compared to those in other countries. The origin and evolution of the dengue virus is complex, and not as clear as of some other viruses. Cases resembling dengue were recorded in the early 1600s in the Caribbean and Panama, with unambiguous descriptions emerging in Africa, Asia, and the Americas in 1779–1788 [79]. DENV was present in Asia in the 1700s before the arrival of Ae. aegypti, suggesting transmission by another mosquito, likely Ae. albopictus [79]. The prevailing belief is that the current four serotypes of the dengue virus originated in Asia. Recent evidence suggests that over 300 years ago, ancestral dengue virus likely existed in Africa [79]. Furthermore, phylogenetic relationships with other flaviviruses, indicate the dengue virus’s affiliation with African lineages, emphasizing a historical transmission by African mosquitoes to primates [79].
Given the significant public health impact of dengue infections in India, understanding genetic diversity, spatial distribution, vaccine effectiveness, and potential new variants is crucial [80]. All four dengue serotypes (DENV-1–4) have been isolated from various parts of India, with DENV-2 and DENV-3 recognized as the most dominant genotypes in terms of disease spread, while DENV-1 has been predominantly observed in recent decades [80]. The genetic diversity and antigenicity of the dengue virus primarily depend on mutations in its envelope protein [80]. Phylogenetic analysis of the envelope protein further subdivides these serotypes into distinct genotypes [81]. A study involving 50 Indian strains, including 19 recombinant strains, indicated that DENV-3 exhibits less sequence diversity compared to other serotypes [81]. Positive selection on several codons correlated with genetic diversity between serotypes, highlighting that amino acid diversity and inter-genotypic recombination are major contributors to antigenicity variation and dengue virus evolution within India [81].
Global studies on Ae. aegypti reveals two primary genetic units: Ae. aegypti formosus in Africa and Ae. aegypti aegypti outside Africa [82]. In contrast, Ae. albopictus, due to insecticide use, adaptability, and human transport, shows limited genetic diversity, spreading rapidly worldwide [83]. Molecular markers like isozymes, restriction fragment length polymorphism, relative afferent pupillary defect, mitochondrial DNA, ribosomal DNA, and microsatellites [83, 84] help to understand genetic diversity in disease-carrying mosquitoes [85]. South India identifies Ae. aegypti haplotypes, revealing global genetic differences [78]. In Delhi, relative afferent pupillary defect analysis shows significant genetic variation linked to urbanization and climate conditions [86]. North-east regions explore genetic diversity and unique Wolbachia infection patterns in Ae. albopictus [87]. Sonitpur district in Assam uses DNA barcoding, unveiling notable Ae. albopictus nucleotide variations [88]. Maharashtra examines COI markers, revealing low genetic variability in Ae. aegypti populations [84]. Rajasthan, using ITS-2 and mitochondrial COI markers, shows low genetic variability in Ae. aegypti’s COI gene [89]. Tamil Nadu’s COX1-based DNA barcoding effectively identifies Ae. albopictus, emphasizing the importance of genetic polymorphism awareness [79]. Comprehensive genetic structure analysis in 22 Indian populations indicates high genetic diversity among Ae. aegypti populations, influenced by human-mediated dispersal and connectivity [17]. Genetic diversity and evolutionary adaptation in mosquitoes are crucial for predicting their future survival, development, and migration patterns [84]. Analyzing genetic variations within mosquito populations and arbovirus populations is essential to identify high-risk areas for effective vector control measures and disease outbreak prevention [86].
Discussion
Our study underscores the significant health impact of arboviruses, particularly dengue, chikungunya, and Zika, in India [12, 19]. The epidemiological complexity of these viruses, influenced by various factors, such as immune selection and cross-reactive antibodies, poses challenges to public health in terms of diagnosis and treatment [23]. The distribution of dengue cases highlights the ongoing threat in states like Kerala, Karnataka, and Maharashtra, emphasizing the need for targeted interventions [12, 19]. The findings also emphasize the global and regional significance of vertical transmission in maintaining and spreading dengue viruses [35, 36, 38-41, 90-93]. Vector surveillance initiatives contribute to a comprehensive understanding of ZIKV and DENV prevalence in Aedes mosquitoes, crucial for effective epidemiological control [47, 48]. Furthermore, the diverse breeding habits of Aedes mosquitoes in India highlight their adaptability to varied environments [59, 60, 67]. Larval indices, such as house index, container index, and breteau index, play a crucial role in assessing dengue epidemiological risk, emphasizing the need for targeted control efforts in specific high-risk areas [60, 94].
Major ecological factors like: Climate change, global trade, travel, urbanization, and population growth have created conducive conditions for dengue vectors and viruses to proliferate [6]. Furthermore, another important factor we cannot ignore for our future vector control measures is insecticide resistance status, though an excellent review was [95] reported in India. The timeline, spanning from the dichloro diphenyltrichloroethane era to the current use of synthetic pyrethroids, reveals a concerning trend of increasing resistance patterns in India [96]. While organophosphates have shown relative efficacy, carbamates, especially temephos, crucial for larval control, exhibit alarming resistance [96]. Spatially, insecticide resistance reports are sporadic, with Delhi and Karnataka recording the highest occurrences [95]. However, comprehensive nationwide surveys are lacking, hindering a holistic understanding and highlighting the urgent necessity for an insecticide resistance management strategy, use of biocontrol agents like copepods, entomopathogenic fungi, and genetically modified mosquitoes show promise, but further research on sustainability and cost-effectiveness is crucial [97]. On the other hand, because of the knowledge gaps highlighted in Table 1 in this review, we signpost future research questions and their probable solutions that will be essential in planning surveillance and control (Figure 4) of Aedes borne diseases in India going forward:
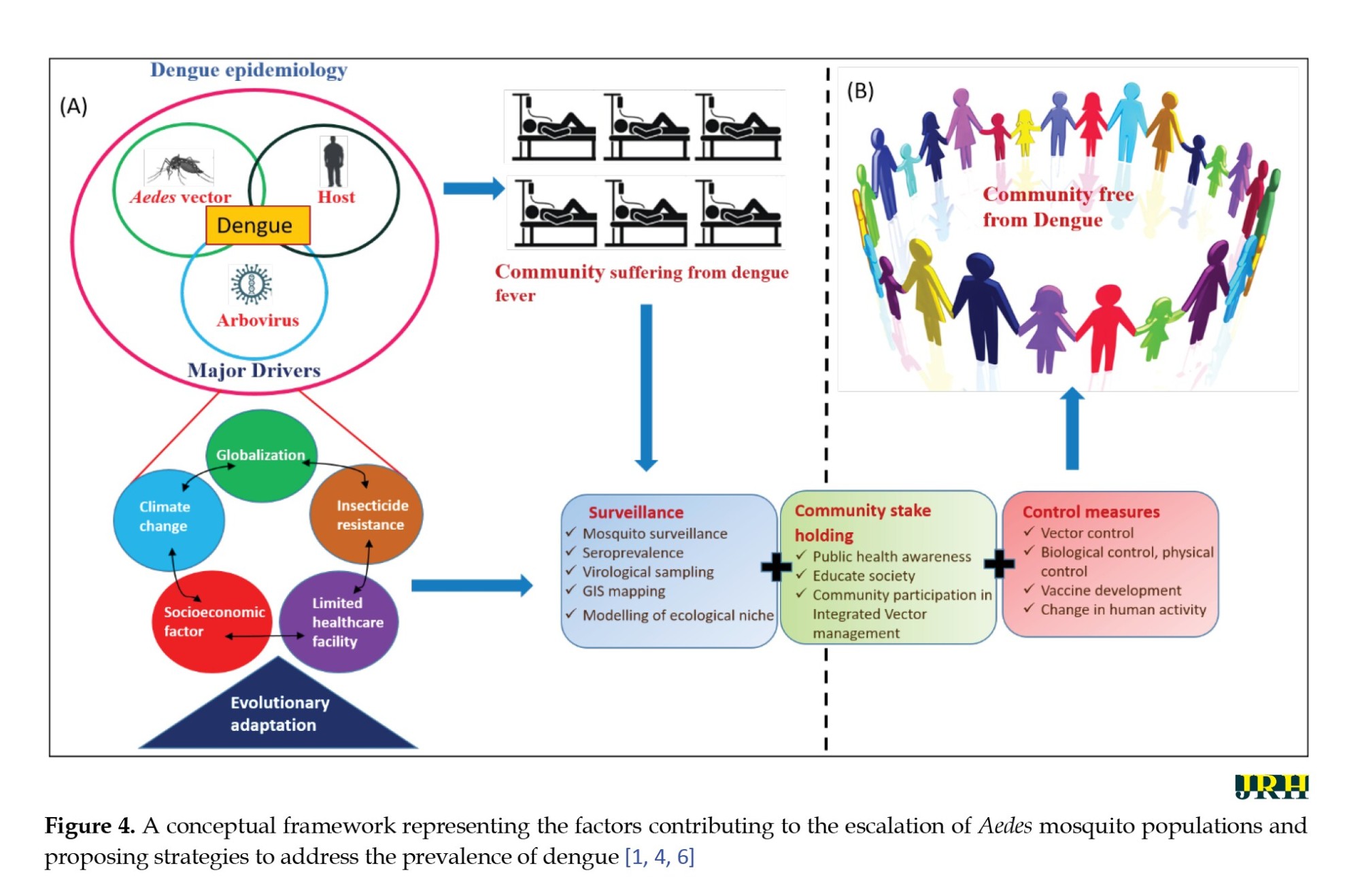
● In-depth epidemiological studies are crucial to identify specific factors contributing to consistently high dengue cases in states [26];
● Collaborations between researchers, healthcare agencies, and vaccine developers, supported by ongoing genomic surveillance, can aid in developing a vaccine that considers the diverse antigen and genotype variations of the arbovirus [26, 28];
● Future strategies should encourage and support research initiatives in regions with limited studies, conducting ecological studies to identify factors influencing arboviral transmission variability and tailoring control measures, accordingly;
● Utilize novel molecular surveillance techniques to understand the genetic and molecular aspects of arbovirus transmission [95];
● Conduct region-specific studies to identify the temporal feeding patterns of Aedes mosquitoes and develop targeted control measures based on these patterns;
Conduct comprehensive studies on the toxicity of sugar baits against non-target organisms and evaluate their residual efficacy in diverse field conditions in India;
● Conduct ecological studies to understand the specific ecological and environmental factors influencing the adaptability of Aedes mosquitoes to diverse breeding habitats in different regions of India;
● Focus on understanding the mechanisms and frequency of mosquito movement through transportation networks, migration routes, and factors supporting long-distance dispersal [95];
● Conduct comprehensive analyses of environmental factors, such as breeding sites, land use patterns, and urbanization levels, to provide valuable insights and explain unexplained variations in genetic structure [95];
● Regular monitoring of insecticide resistance status, resistance mechanisms, and investigation of the impact of organic and other anthropogenic pollutants on the response of Ae. aegypti and Ae. albopictus to insecticides;
● Implementation of awareness initiatives like poster campaigns, volunteer education on source reduction in mosquito breeding, social media campaigns, program implementation for waste management, and essential oil-based mosquito repellent.
Conclusion
This thorough review consolidates information on the behavior, genetics, and virus transmission patterns of mosquitoes, specifically Ae. aegypti and Ae. albopictus, in India. The focus lies on crucial aspects of controlling these vectors. The research has mainly centered on their breeding habits, emphasizing the importance of understanding their feeding patterns, and host preferences for effective disease control. An innovative strategy, using attractive toxic sugar baits, shows promise in controlling mosquitoes by targeting both male and female sugar-feeding behavior. Examining arbovirus transmission dynamics, especially vertical and transovarial transmission in Aedes mosquitoes, reveals regional variations, emphasizing the need for extensive vector surveillance. Genetic diversity and population structure are crucial for assessing adaptability and disease transmission potential. Despite fewer genetic studies in India compared to global efforts, the research underscores significant genetic diversity in Aedes populations, requiring continuous monitoring.
Following the guidelines from the NCVBDC, a comprehensive approach to controlling Aedes mosquitoes are essential. Hospitals should implement prevention guidelines, focusing on source reduction and environmental manipulation. Draining stagnant water from potential breeding sites is crucial. Personal protection measures, including regular use of mosquito nets and repellents, are emphasized. Eco-friendly and cost-effective larvicides, insect growth regulators, and Bacillus thuringiensis israelensis can target larvae, while indoor space spray/fogging is effective during outbreaks. Nodal officers should monitor and coordinate efforts, ensuring effective surveillance and training. Public awareness campaigns further contribute to Aedes mosquito control. Integrating comprehensive vector surveillance with molecular investigations will inform region-specific control strategies, enhancing the precision of interventions and mitigating the impact of mosquito-borne diseases in India.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design and data extraction: Preeti Acharya; Study conduction: Preeti Acharya; Drafting the manuscript: Preeti Acharya; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the laboratory members of Department of Life Sciences and Department of Biotechnology for giving internet assistance during the manuscript preparation.
Reference
Dengue is a predominant arboviral disease transmitted by Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus mosquitoes, pose a significant global threat, contributing to an annual burden of 100–400 million new infections. Approximately 3.83 billion people are at risk worldwide [1, 2]. These vectors also transmit other impactful diseases, such as chikungunya, yellow fever, and Zika infections [3]. The spectrum of dengue infections encompasses mild fever to severe forms, like dengue hemorrhagic fever and dengue shock syndrome, driven by four distinct serotypes (DENV–1, DENV–2, DENV–3, and DENV–4) capable of co-infecting humans [3, 4]. Dengue clinical manifestations include fever, rash, eye pain, arthralgias, myalgias, and hemorrhage [3].
The quantification of the global burden of dengue is challenging due to widespread under-reporting, especially in areas with weaker healthcare systems [5, 6]. Dengue is widespread, affecting over 120 countries, especially in the Americas, Southeast Asia, and the Western Pacific [5, 6]. The global spread of dengue began in the 1980s, reaching various regions, such as the Mediterranean basin, the Americas, and Africa [7]. Brazil has the highest number of reported cases in the Americas, with 4.1 million, while Africa, particularly Burkina Faso, is experiencing a significant increase in the number of cases [7, 8]. Dengue is also reported in Eastern Mediterranean countries, with the emergence of local cases and the expansion of Ae. albopictus in Europe [9]. In the Western Pacific Region, which includes Australia, China, and the Philippines, there are over 500000 reported cases [7]. About 70% of the disease burden is concentrated in Asian countries [2, 5, 6]. South-East Asian nations, like India, Indonesia, Myanmar, Sri Lanka, and Thailand rank among the top 30 countries globally with the highest dengue prevalence, as per the Centers for Disease Control and Prevention 2024 [4, 10].
India’s contribution to global dengue infections is estimated at 34 out of 96 million, significantly contrasting with the reported 12 484 cases to the World Health Organization (WHO), revealing a substantial underreporting gap [2]. However, in India, there is a lack of clear understanding of the distribution of dengue vectors, although their presence has been confirmed in specific regions through local surveillance efforts [11-13]. Major regions in India, are affected by one of the four arboviral infections [14]. Furthermore, a study on the impact of climate change on mosquito abundance in India suggests an increased risk in parts of the Thar Desert and the Upper Himalayas [15]. Despite lower reported dengue cases, there is a high prevalence observed in northeast India and the western coastline, emphasizing the need for heightened monitoring [11]. Ae. aegypti dominates in the Deccan plateau and arid regions of Gujarat and Rajasthan, while Ae. albopictus prevails in the eastern coastline, aligning with reported dengue incidence in Gujarat, Maharashtra, Punjab, and Karnataka [11, 15].
Ae. aegypti and Ae. albopictus showcase adaptability shaped by their evolutionary history and genetic diversity [9, 16]. Ae. aegypti has two subspecies, namely Ae. aegypti aegypti and the ancestral Ae. aegypti formosus, originating from Africa, with the former adapting to urban settings and becoming a primary vector [16]. Ae. albopictus, from Southeast Asia, displays versatility in human-altered environments, spreading worldwide [8]. Behaviorally, Ae. aegypti prefers indoor resting and daytime human feeding, mainly in urban areas and artificial water containers [3, 4]. Conversely, Ae. albopictus, an opportunistic feeder with outdoor resting tendencies, also exhibits anthropophagic behavior [1, 4]. Factors influencing their distribution include urbanization, climate elements (temperature, rainfall, and humidity), and human mobility [1, 4, 6, 11, 17]. Human movement contributes to lower genetic differentiation [15], while changing precipitation patterns impact larval habitats, affecting vector population dynamics [16]. The intricate interplay of these factors contributes to the rapid expansion of vectors and their prevalence worldwide [1, 4, 6, 8, 9, 11, 17].
Despite the increasing number of dengue cases, there is no specific treatment or vaccine available [2, 7, 10, 14]. In India, dengue control relies on different practices, such as indoor spraying, fogging, and personal protection promotion, yet strong evidence of the effectiveness of these interventions is lacking [18, 19]. This underscores the urgent need for tailored control measures. Conventional surveillance often overlooks factors, like seasonal larval abundance fluctuations and non-residential areas within cities, where Aedes mosquitoes thrive [20]. The impact of urbanization, social behavior, and climate change on Aedes mosquitoes in India are not well understood. Recent studies highlight challenges in controlling Ae. aegypti and Ae. albopictus in Western Africa and Thailand due to insufficient research on their behavior [1, 4]. Recognizing the global impact of Aedes-borne diseases, our study addresses knowledge gaps in Aedes mosquito behavior, genetic diversity, and arbovirus transmission in India [1, 4]. To enhance surveillance and guide control efforts, this study pinpoints research gaps in Aedes mosquito behavior in different regions of residential areas and non-residential areas [11, 17, 20].
Methods
This study employed a scoping review approach by following the methodology from [21]. The article selection process involved five steps as follows: Defining the research question, conducting a thorough search for relevant studies, filtering and selecting pertinent articles, synthesizing and interpreting collected data, finally establishing the analytical framework, and reporting the results. Searches were conducted in Google Scholar, Web of Science, Scopus, and PubMed from October to November 2023, focusing on articles published between 2013 and November 2023. We have subdivided the second step of our search strategy into three sub-step processes: 1) An initial search of published studies in Google Scholar, Scopus, PubMed, and Web of Science databases; 2) Utilization of keywords and terms to search additional databases, including Science Direct, relevant government ministry portals (e.g. National Center for Vector Borne Diseases Control (NCVBDC)) and organizations (e.g. WHO and centers for disease control and prevention) by using Google search engine and manual search; 3) Exploration of the reference lists of identified studies for supplementary sources. Appropriate keywords were used to search literature in English language only and articles in other languages were removed. Whenever feasible, we used the preferred reporting items for systematic reviews and meta-analysis criteria [22] for performing and writing the results (Figure 1).

The keywords used to search literature are as follows:
● “Ae. aegypti” AND “dengue” AND “India”. “Aedes albopictus” AND “dengue” AND “India”
● “Ae. aegypti” AND “chikungunya” AND “India”. “Ae. albopictus” AND “chikungunya” AND “India”
● “Ae. aegypti” AND “Zika” AND “India”. “Ae. albopictus” AND “Zika” AND “India”
● “Ae. aegypti” AND “breeding” or “breeding habitat” AND “India”. “Ae. albopictus” AND “breeding” AND “India”
● “Ae. aegypti” AND “feeding preference” or “feeding behavior” or “blood-feeding” AND “India”. “Ae. albopictus” AND “feeding preference” or “feeding behavior” or “blood-feeding” AND “India”
● “Ae. aegypti” AND “genetic diversity” or “genetic structure” or “genetic variability” or AND “India”. “Ae. albopictus” AND “genetic diversity” or “genetic structure” or “genetic variability” or AND “India”
● “Aedes mosquitoes” AND “virus transmission” or “vertical transmission” or “transovarial transmission” AND “India”
The inclusion criteria for this review article were limited to full articles in the English language, concentrating on the status of Aedes borne diseases in India, Aedes mosquito behavior, including feeding behavior, breeding habitats, genetic diversity, arbovirus transmission dynamics in mosquitoes, and patterns of transmission in hosts. The review considered the influence of external factors, like ecological elements (including rainfall, temperature, and humidity) and social factors of urbanization, on mosquito adaptation to changing environments and their increased expansion. Data collection involved research on Ae. aegypti and Ae. albopictus across diverse states in India. The quality of the articles was checked and stored manually. Meanwhile, the exclusion criteria targeted articles that did not focus on the behavior and genetic diversity of Aedes mosquitoes in the Indian context, articles related to mosquitoes other than Aedes and the removal of duplicated or commonly addressed articles. The screening criteria for selecting the objective-related articles included having relevance to the study subject.
Results
Following an initial search and review of titles and abstracts, we identified a total of 102 articles from the online search and an additional 4 articles through manual searching. The details of these articles are outlined in Table 1. To align with the study objective, the extracted data from these articles were categorized into specific themes, including “Aedes mosquito biology and arboviral disease in India,” with sub-categories, such as “arboviral disease in India,” “virus transmission dynamics,” “feeding behavior, host preference, and biting pattern,” “breeding habitat,” and “genetic diversity.”

Arboviral disease in India
In the past two decades, India has witnessed a substantial geographic spread of dengue, experiencing repeated outbreaks and a staggering 11-fold surge in cases [23]. The global dengue outbreak in 2016, coupled with Delhi’s severe outbreak in 2015, underscored the widespread impact of the virus [23]. Dengue’s presence in India dates back to 1950, with the isolation of the first Dengue serotype, DENV-1, in Vellore, Tamilnadu, in 1956, followed by a major outbreak in Calcutta in 1963 [23]. Since the 1960s, outbreaks have been reported across all states and :union: territories, except Ladakh and Lakshadweep [23]. The emergence of dengue hemorrhagic fever intensified outbreaks, particularly in Delhi in 1996 and North India in 2003, highlighting the virus’s variability [24, 25]. According to the NCVBDC, data as of January 2023 (Figures 2A, 2B, and 2C) depict the distribution of reported dengue cases and deaths in India. Subsequent reports in September 2023 indicate a sustained impact of dengue fever, with persistently high cases in states like Kerala, Karnataka, Maharashtra, Odisha, Uttar Pradesh, Assam, Delhi, Telangana, Rajasthan, Tamil Nadu, West Bengal, and Punjab [14, 26].

A nationally representative survey in India unveiled age-specific dengue seroprevalence, estimating an overall prevalence of 48.7%. Variations were observed, ranging from 28.3% in children aged 5–8 years to 56.2% in individuals aged 18–45 years [27]. Geographical differences were evident, with the highest seroprevalence in southern regions (76.9%) and urban areas (70.9%), underscoring the impact of urbanization on dengue incidence [27]. Furthermore, genomic analyses reveal the co-circulation of all four dengue serotypes in different regions of India, with DENV-4 emerging as a dominant serotype in South India [28]. Diagnosis involves detecting NS1 antigen and IgM dengue antibody. Vaccines like TAK-003 are undergoing trials, facing challenges due to diverse antigens and genotype variations [29].
Chikungunya fever in India, caused by Chikungunya virus (CHIKV), is endemic in 24 states and 6 :union: territories [30]. The virus reemerged in 2005, causing a significant outbreak across states, with CHIKV’s adaptability to Ae. albopictus mosquitoes contributing to its rapid spread [31]. Molecular diagnostics, particularly reverse transcription polymerase chain reaction, play a crucial role in diagnosing acute-phase CHIKV infections [30]. Additionally, the Zika virus (ZIKV), with a complex evolutionary history, surfaced in Gujarat and Maharashtra in 1954, reappearing notably in Ahmedabad in 2016, Jaipur in 2018, and Madhya Pradesh in 2021 [32-34]. The continuous emergence of these viruses underscores the importance of robust surveillance, prompt diagnosis, and effective prevention strategies tailored to region-specific strains [33, 34].
Virus transmission dynamics
Arboviruses, transmitted primarily by mosquitoes, utilize horizontal and vertical transmission mechanisms. Horizontal transmission occurs during a blood meal, while vertical transmission involves passing the virus from an infected female mosquito to her offspring through eggs (Figure 3) [35, 36]. Understanding these mechanisms is crucial for studying dengue fever epidemiology. Global studies, including in India, confirm natural vertical transmission of dengue virus in Aedes mosquitoes [37]. Indian studies since 2007 show natural vertical transmission of dengue serotypes, varying globally, with South America and Asia contributing significantly to cases [35]. Africa lacks sufficient research on natural vertical transmission [38].

In India, studies in various states have shed light on the intricate dynamics of arbovirus transmission, particularly in Chennai, emphasizing the role of vertical transmission in maintaining dengue viruses during inter-epidemic periods [39]. Kerala reports natural vertical transmission in Ae. albopictus [40]. Rajasthan investigates dengue-3 virus persistence in successive Ae. aegypti generations, emphasizing re-emergence [41]. Uttar Pradesh reveals natural vertical transmission of dengue virus seen in male Aedes mosquitoes [42]. Delhi’s 2013 outbreak shows Ae. aegypti infection rates contribute to transmission in low and medium-income areas [43]. In Jodhpur, Bhopal, Aedes mosquitoes tested positive for dengue antigen via vertical transmission [44, 45]. Odisha faced rising dengue cases, prompting studies on DENV transmission dynamics [46].
Furthermore, vector surveillance is crucial, as seen in central India’s year-round xenomonitoring [47]. Nationwide surveillance initiated by the Indian Council of Medical Research from 2016 to 2019 across 14 states contributed to our understanding of ZIKV and DENV prevalence in Aedes mosquitoes [48]. Thiruvananthapuram’s intensive vector surveillance during a ZIKV outbreak detected high Aedes mosquito infestation [33].
Feeding behavior, host preference, and biting pattern
Hematophagy, essential for arthropod vector life cycles, underscores the frequent blood feeding of disease vectors like Ae. aegypti and Ae. albopictus, occurring every 2 to 4 days, is crucial for pathogen acquisition and transmission [49]. These mosquitoes exhibit a strong preference for human blood, influenced by environmental factors and host availability [50, 51]. In Asia, where Aedes mosquitoes are prevalent, they serve as competent vectors for various infectious diseases [2]. The body size of these mosquitoes, influenced by temperature and nutrition, impacts their ecology and multiple blood-feeding activities [52]. A study in the South Andaman district found that both Ae. aegypti and Ae. albopictus predominantly feeds on humans, with Ae. albopictus exhibiting a broader host range [53]. In Dehradun City, variations in mosquito abundance based on different habitats and seasons were observed, with Aedes mosquitoes primarily feed on humans, followed by bovines and pigs [54]. Understanding biting rhythms is also crucial, as evidenced by a study in Sivaganga district identifying three main rhythmic patterns among various mosquito species [55]. Additionally, the innovative approach of using attractive toxic sugar baits in India has shown efficacy in reducing mosquito populations by exploiting the sugar-feeding behavior of both male and female mosquitoes [56, 57].
Breeding habitat
India’s diverse landscapes and climates create a complex scenario for Aedes mosquito breeding, key vectors for diseases like dengue [58]. Aedes mosquitoes are adaptable to various habitats, from clean water receptacles to polluted collections like stagnant drain water, tanks, coolers, and containers [59]. Ae. aegypti favors indoor breeding, while Ae. albopictus prefers outdoor areas, like car tires and garbage dumps [59]. Visual and olfactory cues guide their oviposition site selection, considering both chemical and physical factors in the water [58]. Extensive studies across India emphasize Aedes mosquitoes’ adaptability to diverse breeding habitats, with larval indices, like house index, container index, and breteau index, serving as crucial tools for assessing dengue epidemiological risk [60]. In Delhi and Tamil Nadu, significant Aedes breeding dynamics occur in key containers like overhead tanks, enhancing cost-effective and sustainable control efforts [61, 62].
Kerala, susceptible to arboviral diseases, reveals unconventional breeding habitats in crops and brackish water areas [63, 64]. Northern Kerala studies link physicochemical parameters with adult emergence, providing insights into the relationship between water quality and mosquito presence [65]. The adaptability of Ae. albopictus to diverse environments, including discarded automobile tires and water-holding plastic containers have been observed in the Andaman Islands [66]. Various regions, such as Kanyakumari, Tripura, Thrissur, Bengaluru, Odisha, Bihar, Dehradun, and West Bengal identify specific containers, like plastic containers, tires, and water coolers as high-risk areas for Aedes breeding [20, 60, 67-77]. Entomological surveillance international airports underscore varying degrees of receptivity to Aedes vector breeding, emphasizing the importance of consistent monitoring and timely source reduction efforts [76].
Genetic diversity
In India, rising dengue cases necessitate a multidisciplinary approach, integrating morphological, molecular, and distributional data [78]. Our data analysis focuses on two key aspects as follows: First, understanding the origin and evolution of dengue serotypes, and exploring how they adapt to changing environments is one aspect. Secondly, we delve into the genetic variation within Indian Aedes mosquito populations compared to those in other countries. The origin and evolution of the dengue virus is complex, and not as clear as of some other viruses. Cases resembling dengue were recorded in the early 1600s in the Caribbean and Panama, with unambiguous descriptions emerging in Africa, Asia, and the Americas in 1779–1788 [79]. DENV was present in Asia in the 1700s before the arrival of Ae. aegypti, suggesting transmission by another mosquito, likely Ae. albopictus [79]. The prevailing belief is that the current four serotypes of the dengue virus originated in Asia. Recent evidence suggests that over 300 years ago, ancestral dengue virus likely existed in Africa [79]. Furthermore, phylogenetic relationships with other flaviviruses, indicate the dengue virus’s affiliation with African lineages, emphasizing a historical transmission by African mosquitoes to primates [79].
Given the significant public health impact of dengue infections in India, understanding genetic diversity, spatial distribution, vaccine effectiveness, and potential new variants is crucial [80]. All four dengue serotypes (DENV-1–4) have been isolated from various parts of India, with DENV-2 and DENV-3 recognized as the most dominant genotypes in terms of disease spread, while DENV-1 has been predominantly observed in recent decades [80]. The genetic diversity and antigenicity of the dengue virus primarily depend on mutations in its envelope protein [80]. Phylogenetic analysis of the envelope protein further subdivides these serotypes into distinct genotypes [81]. A study involving 50 Indian strains, including 19 recombinant strains, indicated that DENV-3 exhibits less sequence diversity compared to other serotypes [81]. Positive selection on several codons correlated with genetic diversity between serotypes, highlighting that amino acid diversity and inter-genotypic recombination are major contributors to antigenicity variation and dengue virus evolution within India [81].
Global studies on Ae. aegypti reveals two primary genetic units: Ae. aegypti formosus in Africa and Ae. aegypti aegypti outside Africa [82]. In contrast, Ae. albopictus, due to insecticide use, adaptability, and human transport, shows limited genetic diversity, spreading rapidly worldwide [83]. Molecular markers like isozymes, restriction fragment length polymorphism, relative afferent pupillary defect, mitochondrial DNA, ribosomal DNA, and microsatellites [83, 84] help to understand genetic diversity in disease-carrying mosquitoes [85]. South India identifies Ae. aegypti haplotypes, revealing global genetic differences [78]. In Delhi, relative afferent pupillary defect analysis shows significant genetic variation linked to urbanization and climate conditions [86]. North-east regions explore genetic diversity and unique Wolbachia infection patterns in Ae. albopictus [87]. Sonitpur district in Assam uses DNA barcoding, unveiling notable Ae. albopictus nucleotide variations [88]. Maharashtra examines COI markers, revealing low genetic variability in Ae. aegypti populations [84]. Rajasthan, using ITS-2 and mitochondrial COI markers, shows low genetic variability in Ae. aegypti’s COI gene [89]. Tamil Nadu’s COX1-based DNA barcoding effectively identifies Ae. albopictus, emphasizing the importance of genetic polymorphism awareness [79]. Comprehensive genetic structure analysis in 22 Indian populations indicates high genetic diversity among Ae. aegypti populations, influenced by human-mediated dispersal and connectivity [17]. Genetic diversity and evolutionary adaptation in mosquitoes are crucial for predicting their future survival, development, and migration patterns [84]. Analyzing genetic variations within mosquito populations and arbovirus populations is essential to identify high-risk areas for effective vector control measures and disease outbreak prevention [86].
Discussion
Our study underscores the significant health impact of arboviruses, particularly dengue, chikungunya, and Zika, in India [12, 19]. The epidemiological complexity of these viruses, influenced by various factors, such as immune selection and cross-reactive antibodies, poses challenges to public health in terms of diagnosis and treatment [23]. The distribution of dengue cases highlights the ongoing threat in states like Kerala, Karnataka, and Maharashtra, emphasizing the need for targeted interventions [12, 19]. The findings also emphasize the global and regional significance of vertical transmission in maintaining and spreading dengue viruses [35, 36, 38-41, 90-93]. Vector surveillance initiatives contribute to a comprehensive understanding of ZIKV and DENV prevalence in Aedes mosquitoes, crucial for effective epidemiological control [47, 48]. Furthermore, the diverse breeding habits of Aedes mosquitoes in India highlight their adaptability to varied environments [59, 60, 67]. Larval indices, such as house index, container index, and breteau index, play a crucial role in assessing dengue epidemiological risk, emphasizing the need for targeted control efforts in specific high-risk areas [60, 94].
Major ecological factors like: Climate change, global trade, travel, urbanization, and population growth have created conducive conditions for dengue vectors and viruses to proliferate [6]. Furthermore, another important factor we cannot ignore for our future vector control measures is insecticide resistance status, though an excellent review was [95] reported in India. The timeline, spanning from the dichloro diphenyltrichloroethane era to the current use of synthetic pyrethroids, reveals a concerning trend of increasing resistance patterns in India [96]. While organophosphates have shown relative efficacy, carbamates, especially temephos, crucial for larval control, exhibit alarming resistance [96]. Spatially, insecticide resistance reports are sporadic, with Delhi and Karnataka recording the highest occurrences [95]. However, comprehensive nationwide surveys are lacking, hindering a holistic understanding and highlighting the urgent necessity for an insecticide resistance management strategy, use of biocontrol agents like copepods, entomopathogenic fungi, and genetically modified mosquitoes show promise, but further research on sustainability and cost-effectiveness is crucial [97]. On the other hand, because of the knowledge gaps highlighted in Table 1 in this review, we signpost future research questions and their probable solutions that will be essential in planning surveillance and control (Figure 4) of Aedes borne diseases in India going forward:

● In-depth epidemiological studies are crucial to identify specific factors contributing to consistently high dengue cases in states [26];
● Collaborations between researchers, healthcare agencies, and vaccine developers, supported by ongoing genomic surveillance, can aid in developing a vaccine that considers the diverse antigen and genotype variations of the arbovirus [26, 28];
● Future strategies should encourage and support research initiatives in regions with limited studies, conducting ecological studies to identify factors influencing arboviral transmission variability and tailoring control measures, accordingly;
● Utilize novel molecular surveillance techniques to understand the genetic and molecular aspects of arbovirus transmission [95];
● Conduct region-specific studies to identify the temporal feeding patterns of Aedes mosquitoes and develop targeted control measures based on these patterns;
Conduct comprehensive studies on the toxicity of sugar baits against non-target organisms and evaluate their residual efficacy in diverse field conditions in India;
● Conduct ecological studies to understand the specific ecological and environmental factors influencing the adaptability of Aedes mosquitoes to diverse breeding habitats in different regions of India;
● Focus on understanding the mechanisms and frequency of mosquito movement through transportation networks, migration routes, and factors supporting long-distance dispersal [95];
● Conduct comprehensive analyses of environmental factors, such as breeding sites, land use patterns, and urbanization levels, to provide valuable insights and explain unexplained variations in genetic structure [95];
● Regular monitoring of insecticide resistance status, resistance mechanisms, and investigation of the impact of organic and other anthropogenic pollutants on the response of Ae. aegypti and Ae. albopictus to insecticides;
● Implementation of awareness initiatives like poster campaigns, volunteer education on source reduction in mosquito breeding, social media campaigns, program implementation for waste management, and essential oil-based mosquito repellent.
Conclusion
This thorough review consolidates information on the behavior, genetics, and virus transmission patterns of mosquitoes, specifically Ae. aegypti and Ae. albopictus, in India. The focus lies on crucial aspects of controlling these vectors. The research has mainly centered on their breeding habits, emphasizing the importance of understanding their feeding patterns, and host preferences for effective disease control. An innovative strategy, using attractive toxic sugar baits, shows promise in controlling mosquitoes by targeting both male and female sugar-feeding behavior. Examining arbovirus transmission dynamics, especially vertical and transovarial transmission in Aedes mosquitoes, reveals regional variations, emphasizing the need for extensive vector surveillance. Genetic diversity and population structure are crucial for assessing adaptability and disease transmission potential. Despite fewer genetic studies in India compared to global efforts, the research underscores significant genetic diversity in Aedes populations, requiring continuous monitoring.
Following the guidelines from the NCVBDC, a comprehensive approach to controlling Aedes mosquitoes are essential. Hospitals should implement prevention guidelines, focusing on source reduction and environmental manipulation. Draining stagnant water from potential breeding sites is crucial. Personal protection measures, including regular use of mosquito nets and repellents, are emphasized. Eco-friendly and cost-effective larvicides, insect growth regulators, and Bacillus thuringiensis israelensis can target larvae, while indoor space spray/fogging is effective during outbreaks. Nodal officers should monitor and coordinate efforts, ensuring effective surveillance and training. Public awareness campaigns further contribute to Aedes mosquito control. Integrating comprehensive vector surveillance with molecular investigations will inform region-specific control strategies, enhancing the precision of interventions and mitigating the impact of mosquito-borne diseases in India.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design and data extraction: Preeti Acharya; Study conduction: Preeti Acharya; Drafting the manuscript: Preeti Acharya; Review, editing and final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the laboratory members of Department of Life Sciences and Department of Biotechnology for giving internet assistance during the manuscript preparation.
Reference
- Egid BR, Coulibaly M, Dadzie SK, Kamgang B, McCall PJ, Sedda L, et al. Review of the ecology and behavior of aedes aegypti and aedes albopictus in Western Africa and implications for vector control. Current Research in Parasitology & Vector-Borne Diseases. 2022; 2:100074. [DOI:10.1016/j.crpvbd.2021.100074] [PMID]
- World health organization (WHO). Dengue and severe dengue. Geneva: WHO; 2014. [Link]
- Meena AR. Review on surveillance and bionomics of (aedes mosquitoes) dengue vectors. International Journal of Entomology Research. 2020; 5(1):78-81. [Link]
- Ahebwa A, Hii J, Neoh KB, Chareonviriyaphap T. Aedes aegypti and aedes albopictus (Diptera: Culicidae) ecology, biology, behaviour, and implications on arbovirus transmission in Thailand: Review. One Health (Amsterdam, Netherlands). 2023; 16:100555. [DOI:10.1016/j.onehlt.2023.100555] [PMID]
- Banerjee I, Robinson J. Dengue on the rise 2022-2023: A warning for Southern Asia. Research Developments in Medicine and Medical Science. 2023; 1:153-63. [DOI:10.9734/bpi/rdmms/v1/4674B]
- Palaniyandi M, Sharmila T, Manivel P, Thirumalai P, Anand PH. Mapping the geographical distribution and seasonal variation of dengue and chikungunya vector mosquitoes (aedes aegypti and aedes albopictus) in the epidemic hotspot regions of India. Journal of Applied Ecology and Environmental Sciences. 2020; 8(6)428-40. [DOI:10.12691/aees-8-6-15]
- World Health Organization (WHO). Dengue - global situation. Geneva: WHO; 2023. [Link]
- Battaglia V, Agostini V, Moroni E, Colombo G, Lombardo G, Rambaldi Migliore N, et al. The worldwide spread of aedes albopictus: New insights from mitogenomes. Frontiers in Genetics. 2022; 13:931163. [DOI:10.3389/fgene.2022.931163] [PMID]
- Zhao M, Ran X, Bai Y, Ma Z, Gao J, Xing D, et al. Genetic diversity of aedes aegypti and aedes albopictus from cohabiting fields in Hainan Island and the Leizhou Peninsula, China. Parasites & Vectors. 2023; 16(1):319. [DOI:10.1186/s13071-023-05936-5] [PMID]
- LSánchez-González L, Adams L, Paz-Bailey G. Travel-associated infections & diseases. In: Abanyie F, Abe K, Acosta A, Adams L, Ali Ibne, Allen Tchoukalov J, editors. Dengue: CDC yellow book 2024. Puerto Rico: Centers for Disease Control and Prevention (CDC); 2024. [Link]
- Hussain SSA, Dhiman RC. Distribution expansion of dengue vectors and climate change in India. Geohealth. 2022; 6(6):e2021GH000477. [DOI:10.1029/2021GH000477] [PMID]
- Soni M, Bhattacharjee CK, Khan SA, Dutta P. DNA barcoding as a complementary approach for species identification from dengue endemic regions of North East India. International Journal Mosquito Research. 2018; 5:46-52. [Link]
- Shil P, Sapkal GN, Patil AA, Gunjal SN, Sudeep AB. Meteorological parameters and seasonal variability of mosquito population in Pune Urban Zone, India: A year-round study, 2017. Journal of Mosquito Research. 2018; 8(3):18-28. [DOI:10.5376/jmr.2018.08.0003]
- National Center for Vector Borne Disease Control (NCVBDC). Operational guidelines for prevention and control of aedes mosquitoes in hospital settings. Delhi: NCVBDC; 2022. [Link]
- Sarkar S, Gangare V, Singh P, Dhiman RC. Shift in potential malaria transmission areas in India, using the fuzzy-based climate suitability malaria transmission (FCSMT) model under changing climatic conditions. International Journal of Environmental Research and Public Health. 2019; 16(18):3474. [DOI:10.3390/ijerph16183474] [PMID]
- Longbottom J, Walekhwa AW, Mwingira V, Kijanga O, Mramba F, Lord JS. Aedes albopictus invasion across Africa: the time is now for cross-country collaboration and control. The Lancet Global Health. 2023; 11(4):e623-8. [DOI:10.1016/S2214-109X(23)00046-3] [PMID]
- Sumitha MK, Kalimuthu M, Kumar MS, Paramasivan R, Kumar NP, Sunish IP, et al. Genetic differentiation among aedes aegypti populations from different eco-geographical zones of India. PLoS Neglected Tropical Diseases. 2023; 17(7):e0011486. [DOI:10.1371/journal.pntd.0011486] [PMID]
- Aubry F, Dabo S, Manet C, Filipović I, Rose NH, Miot EF, et al. Enhanced zika virus susceptibility of globally invasive aedes aegypti populations. Science. 2020; 370(6519):991-6. [DOI:10.1126/science.abd3663] [PMID]
- National Center for Vector Borne Disease Control (NCVBDC). Handbook on prevention and control of Dengue in School. Delhi: NCVBDC; 2019. [Link]
- Dharmamuthuraja D, P DR, Lakshmi MI, Isvaran K, Ghosh SK, Ishtiaq F. Determinants of aedes mosquito larval ecology in a heterogeneous urban environment- A longitudinal study in Bengaluru, India. PLoS Neglected Tropical Diseases. 2023; 17(11):e0011702. [DOI:10.1371/journal.pntd.0011702] [PMID]
- Arksey H, O'malley L. Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology. 2005; 8(1):19-32. [DOI:10.1080/1364557032000119616]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine. 2009; 151(4):264-9. [DOI:10.7326/0003-4819-151-4-200908180-00135] [PMID]
- Prajapati AK, Singh NP, Jain PK, Srivastava DK, Prajapati R. Dengue in India: An overview. National Journal of Community Medicine. 2022; 13(01):49-57. [DOI:10.5455/njcm.20211204035455]
- Centers for Disease Control and Prevention (CDC). Dengue and dengue hemorrhagic fever. Puerto Rico: CDC; 2008. [Link]
- Mondal N. The resurgence of dengue epidemic and climate change in India. Lancet (London, England). 2023; 401(10378):727-8. [DOI:10.1016/S0140-6736(23)00226-X] [PMID]
- Singh N, Singh AK, Kumar A. Dengue outbreak update in India: 2022. Indian Journal of Public Health. 2023; 67(1):181-3. [DOI:10.4103/ijph.ijph_1517_22]
- Wilder-Smith, Annelies, and Priscilla Rupali. Estimating the dengue burden in India. Lancet Glob Health. 2019: e988-9. [DOI: 10.1016/S2214-109X(19)30249-9] [PMID]
- Jagtap S, Pattabiraman C, Sankaradoss A, Krishna S, Roy R. Evolutionary dynamics of dengue virus in India. PLoS Pathog. 2023; 19(4):e1010862. [DOI:10.1371/journal.ppat.1010862] [PMID]
- Sankaradoss A, Jagtap S, Nazir J, Moula SE, Modak A, Fialho J, et al. Immune profile and responses of a novel dengue DNA vaccine encoding an EDIII-NS1 consensus design based on Indo-African sequences. Molecular Therapy. 2022; 30(5):2058-77. [DOI:10.1016/j.ymthe.2022.01.013] [PMID]
- Acevedo N, Waggoner J, Rodriguez M, Rivera L, Landivar J, Pinsky B, et al. zika virus, chikungunya virus, and dengue virus in cerebrospinal fluid from adults with neurological manifestations, Guayaquil, Ecuador. Frontiers in Microbiology. 2017; 8:42. [DOI:10.3389/fmicb.2017.00042] [PMID]
- Singh, Raj Kumar, and Sukhvir Singh. Prevalence of aedes Mosquitoes during the First Outbreak of zika in Jaipur City, India. Journal of Communicable Diseases. 2019; 51(3):33-9. [DOI:10.24321/0019.5138.201924]
- Sasi MS, Rajendran R, Meenakshy V, Suresh T, Heera Pillai R, Dilip Kumar T, et al. Study on vector dynamics of zika virus outbreak in thiruvananthapuram, Kerala, India. International Journal of Current Microbiology and Applied Sciences. 2021; 10(12):54-71. [DOI:10.20546/ijcmas.2021.1012.008]
- Singh H, Singh OP, Akhtar N, Sharma G, Sindhania A, Gupta N, Valecha N. First report on the transmission of zika virus by aedes (stegomyia) aegypti (L.)(Diptera: Culicidae) during the 2018 zika outbreak in India. Acta Tropica. 2019; 199:105114. [DOI:10.1016/j.actatropica.2019.105114] [PMID]
- da Costa CF, Dos Passos RA, Lima JBP, Roque RA, de Souza Sampaio V, Campolina TB, et al. Transovarial transmission of DENV in aedes aegypti in the Amazon basin: A local model of xenomonitoring. Parasites & Vectors. 2017; 10(1):249. [DOI:10.1186/s13071-017-2194-5] [PMID]
- Agboli E, Leggewie M, Altinli M, Schnettler E. Mosquito-specific viruses-transmission and interaction. Viruses. 2019; 11(9):873. [DOI:10.3390/v11090873] [PMID]
- Grunnill M, Boots M. How important is vertical transmission of dengue viruses by mosquitoes (Diptera: Culicidae)? Journal of Medical Entomology. 2016; 53(1):1-19. [DOI:10.1093/jme/tjv168] [PMID]
- Thenmozhi, Velayutham, et al. Natural vertical transmission of dengue virus in aedes albopictus (Diptera: Culicidae) in Kerala, a southern Indian state. Japanese Journal of Infectious Diseases. 2007; 60(5):245. [DOI:10.7883/yoken.JJID.2007.245] [PMID]
- Hartanti MD, Suryani S, Tirtadjaja IA. Dengue virus transovarial transmission by aedes aegypti. Universa Medicina. 2010; 29(2):65-70. [DOI:10.18051/UnivMed.2010.v29.65-70]
- Edillo FE, Sarcos JR, Sayson SL. Natural vertical transmission of dengue viruses in aedes aegypti in selected sites in Cebu City, Philippines. Journal of Vector Ecology. 2015; 40(2):282-91. [DOI:10.1111/jvec.12166] [PMID]
- Angel B, Joshi V. Distribution and seasonality of vertically transmitted dengue viruses in aedes mosquitoes in arid and semi-arid areas of Rajasthan, India. Journal of Vector Borne Diseases. 2008; 45(1):56. [PMID]
- Arunachalam N, Tewari SC, Thenmozhi V, Rajendran R, Paramasivan R, Manavalan R, et al. Natural vertical transmission of dengue viruses by aedes aegypti in Chennai, Tamil Nadu, India. Indian Journal of Medical Research. 2008; 127(4):395-407. [Link]
- Vikram K, Nagpal BN, Pande V, Srivastava A, Saxena R, Singh H, et al. Detection of dengue virus in individual aedes aegypti mosquitoes in Delhi, India. Journal of Vector Borne Diseases. 2015; 52(2):129-33. [DOI:10.4103/0972-9062.159496] [PMID]
- Joshi V, Sharma RC, Sharma Y, Adha S, Sharma K, Singh H, et al. Importance of socioeconomic status and tree holes in distribution of aedes mosquitoes (Diptera: Culicidae) in Jodhpur, Rajasthan, India. Journal of Medical Entomology. 2014; 43(2):330-6. [DOI:10.1603/0022-2585(2006)043[0330:IOSSAT]2.0.CO;2] [PMID]
- Sarma DK, Rathod L, Mishra S, Das D, Agarwal A, Sharma G, et al. Molecular surveillance of dengue virus in field-collected aedes mosquitoes from Bhopal, central India: Evidence of circulation of a new lineage of serotype 2. Frontiers in Microbiology. 2023; 14:1260812. [DOI:10.3389/fmicb.2023.1260812] [PMID]
- Mohanty I, Rath A, Pradhan N, Panda BB, Mohapatra PK, Hazra RK. Prevalence and transmission potential of Wolbachia in aedes albopictus population circulating in endemic coastal districts of Odisha, India. Journal of Vector Borne Diseases. 2021; 58(4):297-305. [DOI:10.4103/0972-9062.313967] [PMID]
- Chand G, Godbole S, Shivlata L, Sahare LK, Ukey M, Kaushal LS, et al. Molecular xenomonitoring of Dengue, Chikungunya and zika infections: A year-round study from two dengue endemic districts of Central India. Journal of Vector Borne Diseases. 2021; 58(2):135-40. [DOI:10.4103/0972-9062.321753] [PMID]
- Kumar NP, Kumar A, Panneer D, Abidha S, Muthukumaravel S, Sankari T, et al. Nation-wide vector surveillance on zika and dengue did not indicate transmission of the American lineage-pandemic zika virus in India. International Journal of Infectious Diseases. 2021; 113:119-24. [DOI:10.1016/j.ijid.2021.09.074] [PMID]
- Brackney DE, LaReau JC, Smith RC. Frequency matters: How successive feeding episodes by blood-feeding insect vectors influences disease transmission. PLoS Pathogens. 2021; 17(6):e1009590. [DOI:10.1371/journal.ppat.1009590] [PMID]
- Kumar A, Arya H, Singh AP, Singh S, Sharma S, Singh K. Surveillance of aedes diversity, seasonal prevalence and habitat characterization in Bulandshahr, Utter Pradesh, India. Journal of Science Innovations and Nature of Earth. 2023; 3(1):04-10. [DOI:10.59436/https://jsiane.com/archives3/1/62]
- Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annual Review of Entomology. 2013; 58:433-53. [DOI:10.1146/annurev-ento-120811-153618] [PMID]
- Fikrig K, Harrington LC. Understanding and interpreting mosquito blood feeding studies: The case of aedes albopictus. Trends in Parasitology. 2021; 37(11):959-75. [DOI:10.1016/j.pt.2021.07.013] [PMID]
- Sivan A, Shriram AN, Sunish IP, Vidhya PT. Host-feeding pattern of aedes aegypti and aedes albopictus (Diptera: Culicidae) in heterogeneous landscapes of South Andaman, Andaman and Nicobar Islands, India. Parasitology Research. 2015; 114(9):3539-46. [DOI:10.1007/s00436-015-4634-5] [PMID]
- Mondal R, Pemola Devi N, Bhattacharya S. Seasonal. Seasonal prevalence and host preference of some medically important aedes species of Doon Valley, India. Journal of Communicable Diseases. 2021; 53(3):96-103. [DOI:10.24321/0019.5138.202144]
- Paramasivan R, Philip SP, Selvaraj PR. Biting rhythm of vector mosquitoes in a rural ecosystem of South India. International Journal of Mosquito Research. 2015; 2(3):106-13. [Link]
- Kumar G, Gupta SK, Pasi S. Attractive toxic sugar baits: a magic bullet for control of malaria and dengue in urban settings of India? Journal of Vector Borne Diseases. 2023; 60(3):340-1. [DOI:10.4103/0972-9062.374242] [PMID]
- Kumar G, Sharma A, Dhiman RC. Laboratory evaluation of the efficacy of boric acid containing toxic sugar baits against Anopheles culicifacies, An. stephensi and aedes aegypti mosquitoes. Journal of Vector Borne Diseases. 2022; 59(1):52-6. [DOI:10.4103/0972-9062.331414] [PMID]
- Rao MR, Padhy RN. A comprehensive study on the larvae of aedes species in Angul district of Odisha, India: An approach to determine their habitat and prevalence in association with the ecology. Journal of Pharmaceutical and Biological Science. 2014; 9(4):98-109. [DOI:10.9790/3008-094198109]
- Panigrahi SK. Research article survey of aedes mosquito breeding sites, density and pattern of distribution: An approach to manage dengue outbreaks in Bhawanipatna Town, Odisha, India. Research Journal of Pharmacy and Life Sciences. 2021; 2(3):46-65. [Link]
- Gould DJ, Mount GA, Scanlon JE, Ford HR, Sullivan MF. Ecology and control of dengue vectors on an island in the Gulf of Thailand. Journal of Medical Entomology. 1970; 7(4):499-508. [DOI:10.1093/jmedent/7.4.499] [PMID]
- Dinesh DS, Singh H, Topno RK, Kumar V, Kesari S, Singh SP, et al. Surveillance of breeding sites of dengue vector following the floods in an urban area of Patna, Bihar, India. Dengue Bulletin. 2020; 41:85-95. [Link]
- Wilson JJ, Sevarkodiyone SP. Breeding preference ratio of dengue and chikungunya vectors in certain rural villages of Virudhunagar district, Tamil Nadu, South India. World Applied Sciences Journal. 2014; 30:787-91. [Link]
- Vikram K, Nagpal BN, Pande V, Srivastava A, Gupta SK, Anushrita VP, et al. Comparison of ae. aegypti breeding in localities of different socio-economic groups of Delhi, India. International Journal of Mosquito Research. 2015; 2(3):83-8. [Link]
- Shamna AK, Vipinya C, Sumodan PK. Detection of aedes albopictus (Diptera: Culicidae) breeding in brackish water habitats in coastal Kerala, India and its implications for dengue scenario in the state. International Journal Entomology Research. 2022; 7:214-6. [Link]
- Jiji PV, Harilal CC. Seasonal changes in the habitat characteristics on survivability and adult emergence of aedes albopictus (Skuse) from rural and urban environments of northern Kerala, India. International Journal of Mosquito Research 2022; 9(6):46-55. [DOI:10.22271/23487941.2023.v10.i1a.661]
- Rajendran R, Anusree SB, Regu K. Seasonal variation and breeding preference of aedes albopictus in one of the coastal districts of Kerala. International Journal of Mosquito Research. 2019; 7(4):78-82. [Link]
- Shriram AN, Sivan A, Sugunan AP. Spatial distribution of aedes aegypti and aedes albopictus in relation to geo-ecological features in South Andaman, Andaman and Nicobar Islands, India. Bulletin of Entomological Research. 2018; 108(2):166-174. [DOI:10.1017/S0007485317000645] [PMID]
- Nagpala BN, Guptaa SK, Shamima A, Vikrama K, Anushritaa HS, Saxenaa R, et al. Identification of key containers of aedes breeding- A cornerstone to control strategies of dengue in Delhi, India. Dengue Bulletin. 2016; 39:87-99. [Link]
- Biswas S, Rajkonwar J, Nirmolia T, Jena SR, Sarkar U, Bhattacharyya DR, et al. First report of rubber collection bowls & plastic and bamboo water containers as the major breeding source of Ae. albopictus with the indigenous transmission of Dengue and Chikungunya in rural forested malaria-endemic villages of Dhalai District, Tripura, India: The importance of molecular identification. Biomedicines. 2023; 11(8):2186. [DOI:10.3390/biomedicines11082186] [PMID]
- Vaz C, Harikumar A, Mundodan JM, Rafi M, Saju CR. Mosquito density in rural Kerala: A study on the trend of aedes larval indices over monsoon in a rural area of Thrissur district, India. International Journal of Community Medicine and Public Health. 2019; 6(10):4528-32. [DOI:10.18203/2394-6040.ijcmph20194524]
- Kumar NP, Saini P, Samuel P, Ajithlal P, Suresh A, Mathew J, et al. ZIKV outbreak in Thiruvananthapuram, Kerala, India, 2021- A primary report. Research Square. Preprint. 2021; 1-8. [DOI:10.21203/rs.3.rs-900208/v1]
- Basra GK, Rohilla S, Singh S. Prevalence of aedes aegypti in Shahdara Zone, Delhi, India. International Journal of Mosquito Research. 2021; v(4):543. [DOI:10.22271/23487941.2021.v8.i4a.543]
- Roy A, Kaliyamoorthy M, De A, Sunish IP, Sahu VK, Takuya TK. Spatial and temporal variations in stegomyia indices of arboviral vectors (aedes aegypti and Ae. albopictus) in an urban agglomeration of Port Blair, Andaman and Nicobar Islands India. Journal of Medical Arthropodology and Public Health. 2021; 1(2):9-27. [Link]
- Mandla S, Illendula S, Mandari A, Bashaboyina R. Identification and seasonal distribution of mosquito species in Telangana state, India. Journal of Entomological Research. 2021; 45(1):27-32. [DOI:10.5958/0974-4576.2021.00004.9]
- Singh S, Purohit MK. Seasonal fluctuation and breeding preferences of aedes aegypti (Diptera: Cuclicidae) in selected localities of Dehradun, Uttarakhand. Journal Global Values. 2023; XIV(Special Issue):469-72. [Link]
- Paramanik M. A preliminary investigation on the container breeding mosquito aedes in a non-endemic municipal city of West Bengal, India. International Journal of Entomology Research. 2023; 8(2):37-40. [Link]
- Sharma SN, Singh SK. Assessment of the receptivity of dengue vector breeding through pre-and post-monsoon vector surveillance at some international airports in India. Journal of Communicable Diseases. 2021; 53(3):16-22. [DOI:10.24321/0019.5138.202135]
- Selvan PS, Jebanesan A, Divya G, Ramesh V. Diversity of mosquitoes and larval breeding preference based on physico-chemical parameters in Western Ghats, Tamilnadu, India. Asian Pacific Journal of Tropical Disease. 2015; 5:S59-66. [DOI:10.1016/S2222-1808(15)60858-1]
- Tyagi V, Kumar Sharma A, Veer V. Evidence of genetic polymorphism in aedes aegypti population from Delhi, India. International Journal of Mosquito Research. 2017; 58:58-63. [Link]
- Powell, JR. Mosquito-borne human viral diseases: Why aedes aegypti? The American Journal of Tropical Medicine and Hygiene. 2018; 98(6):1563-5. [DOI:10.4269/ajtmh.17-0866] [PMID]
- Alagarasu K, Patil JA, Kakade MB, More AM, Yogesh B, Newase P, et al. Serotype and genotype diversity of dengue viruses circulating in India: A multi-centre retrospective study involving the virus research diagnostic laboratory network in 2018. International Journal of Infectious Diseases. 2021; 111:242-52. [DOI:10.1016/j.ijid.2021.08.045] [PMID]
- Rexliene J, Sridhar J. Genetic diversity and evolutionary dynamics of dengue isolates from India. Virusdisease. 2019; 30(3):354-9. [DOI:10.1007/s13337-019-00538-1] [PMID]
- Vadivalagan C, Karthika P, Murugan K, Panneerselvam C, Paulpandi M, Madhiyazhagan P, et al. Genetic deviation in geographically close populations of the dengue vector aedes aegypti (Diptera: Culicidae): Influence of environmental barriers in South India. Parasitology Research. 2016; 115(3):1149-60. [DOI:10.1007/s00436-015-4847-7] [PMID]
- Manyu S, Elanchezhiyan C, Sivasankaran K, Basker P. Molecular identification of aedes albopictus (Skuse, 1894) of Chidambaram strain from inferred mitochondrial gene COI in dengue infested Sentinel site, Tamil Nadu, India. International Journal of Mosquito Research. 2022; 9(4):47-52. [DOI:10.22271/23487941.2022.v9.i4a.622]
- Aguirre-Obando OA, Navarro-Silva MA. How much is known about the genetic diversity of the Asian tiger mosquito? A systematic review. Revista de la Universidad Industrial de Santander. Salud. 2017; 49(3):422-37. [DOI:10.18273/revsal.v49n3-2017001]
- Gloria-Soria A, Ayala D, Bheecarry A, Calderon-Arguedas O, Chadee DD, Chiappero M, et al. Global genetic diversity of aedes aegypti. Molecular Ecology. 2016; 25(21):5377-95. [DOI:10.1111/mec.13866] [PMID]
- Vinayagam S, Nirmolia T, Chetry S, Kumar NP, Saini P, Bhattacharyya DR, et al. Molecular evidence of wolbachia species in wild-caught aedes albopictus and aedes aegypti mosquitoes in four states of Northeast India. Journal of Tropical Medicine. 2023; 2023:6678627. [DOI:10.1155/2023/6678627] [PMID]
- Das M, Das MK, Dutta P. Genetic characterization and molecular phylogeny of aedes albopictus (Skuse) species from Sonitpur district of Assam, India based on COI and ITS1 genes. Journal of Vector Borne Diseases. 2016; 53(3):240-7. [DOI:10.4103/0972-9062.191342] [PMID]
- Gupta K, Dhawan R, Kajla M, Kumar S, Jnanasiddhy B, Singh NK, et al. Molecular identification of aedes aegypti mosquitoes from Pilani region of Rajasthan, India. Journal of Vector Borne Diseases. 2016; 53(2):149-55. [DOI:10.4103/0972-9062.184842] [PMID]
- Kumar SS, Evans DA, Muthulakshmi K, DilipKumar T, Pillai RH, Nair RR, et al. Distribution of aedes aegypti and aedes albopictusin different eco zones of Thiruvananthapuram city with special reference to dengue viremia in humans. Entomon. 2018; 43(4):223-30. [DOI:10.33307/entomon.v43i4.402]
- Jousset FX. Geographic aedes aegypti strains and dengue-2 virus: susceptibility, ability to transmit to vertebrate and transovarial transmission. Annales de l’Institut Pasteur/Virologie. 1981; 132(3):357-70. Elsevier Masson. [DOI:10.1016/S0769-2617(81)80006-8]
- Mitchell CJ, Miller BR. Vertical transmission of dengue viruses by strains of aedes albopictus recently introduced into Brazil. Journal of the American Mosquito Control Association. 1990; 6(2):251-3. [PMID]
- Ferreira-de-Lima VH, Lima-Camara TN. Natural vertical transmission of dengue virus in aedes aegypti and aedes albopictus: a systematic review. Parasites & Vectors. 2018; 11(1):77. [DOI:10.1186/s13071-018-2643-9] [PMID]
- Thenmozhi V, Tewari SC, Manavalan R, Balasubramanian A, Gajanana A. Natural vertical transmission of dengue viruses in aedes aegypt in southern India. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000; 94(5):507. [DOI:10.1016/S0035-9203(00)90067-1] [PMID]
- Sumodan PK. Unconventional breeding habitats of aedes (stegomyia) albopictus (skuse) related to agriculture crops in Kerala, India: A review. International Journal of Mosquito Research. 2019; 6(6):113-15. [Link]
- Sumitha MK, Kalimuthu M, Senthil MK, Paramasivan R, Kumar A, Gupta B. Status of insecticide resistance in the dengue vector aedes aegypti in India: A review. Journal of Vector Borne Diseases. 2023; 60(2):116-24. [DOI:10.4103/0972-9062.361174] [PMID]
- Campos KB, Martins AJ, Rodovalho CM, Bellinato DF, Dias LDS, Macoris MLDG, et al. Assessment of the susceptibility status of aedes aegypti (Diptera: Culicidae) populations to pyriproxyfen and malathion in a nation-wide monitoring of insecticide resistance performed in Brazil from 2017 to 2018. Parasites & Vectors. 2020; 13(1):531. [DOI:10.1186/s13071-020-04406-6] [PMID]
- Gan SJ, Leong YQ, Bin Barhanuddin MFH, Wong ST, Wong SF, Mak JW, et al. Dengue fever and insecticide resistance in aedes mosquitoes in Southeast Asia: A review. Parasites & Vectors. 2021; 14(1):315. [DOI:10.1186/s13071-021-04785-4] [PMID]
- Gandhi R, Yadav KK, Patil PB, Bihani P, Char B, Dasgupta SK, et al. Molecular analysis of mitochondrial cytochrome oxidase I gene of aedes aegypti L. mosquitoes. Journal of Asia-Pacific Entomology. 2020; 23(1):51-9. [DOI:10.1016/j.aspen.2019.10.006]
- Sharma RS, Kaul SM, Sokhay J. Seasonal fluctuations of dengue fever vector, aedes aegypti (Diptera: Culicidae) in Delhi, India. The Southeast Asian Journal of Tropical Medicine and Public Health. 2005; 36(1):186-90. [PMID]
- Borah H, Bora DS. Ecological and social determinants of aedes aegypti and aedes albopictus larval habitat in northeastern India. International Journal of Mosquito Research. 2022; 9(1):47-55. [DOI:10.22271/23487941.2022.v9.i1a.580]
- Meena, A. R. Distribution of aedes aegypti and aedes albopictus from Udaipur District (Rajasthan) India. Journal of Emerging Technologies and Innovative Research. 2019; 6(1):636-41. [Link]
- Meena, A. R, & Choudhary, N. L. Container breeding preference of aedes albopictus in urban environment. International Journal of Mosquito Research. 2019; 6(5):44-7. [Link]
- Marin G, Vincent S, Selvakumar S, Arivoli S, Tennyson S. Surveillance of the Asian tiger mosquito aedes albopictus Skuse 1894 (Diptera: Culicidae) the dengue vector in rubber plantations of Kanyakumari district, Tamil Nadu, India. International Journal of Mosquito Research. 2020; 7(4):127-33. [Link]
Type of Study: Review Article |
Subject:
● Disease Control
Received: 2023/12/5 | Accepted: 2024/01/22 | Published: 2024/07/1
Received: 2023/12/5 | Accepted: 2024/01/22 | Published: 2024/07/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |