Volume 15, Issue 3 (May & June 2025)
J Research Health 2025, 15(3): 303-310 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Meraji M, Bameri H, Ebnehoseini Z, Mahmoodian S S, Ramezanghorbani N. Assessing the Usability of the National Drug Reaction Reporting System: A Heuristic Analysis of User Experience. J Research Health 2025; 15 (3) :303-310
URL: http://jrh.gmu.ac.ir/article-1-2479-en.html
URL: http://jrh.gmu.ac.ir/article-1-2479-en.html
Marziyhe Meraji1 

 , Haniyeh Bameri1
, Haniyeh Bameri1 

 , Zahra Ebnehoseini2
, Zahra Ebnehoseini2 

 , Sanaz Sadat Mahmoodian1
, Sanaz Sadat Mahmoodian1 

 , Nahid Ramezanghorbani3
, Nahid Ramezanghorbani3 




 , Haniyeh Bameri1
, Haniyeh Bameri1 

 , Zahra Ebnehoseini2
, Zahra Ebnehoseini2 

 , Sanaz Sadat Mahmoodian1
, Sanaz Sadat Mahmoodian1 

 , Nahid Ramezanghorbani3
, Nahid Ramezanghorbani3 


1- Department of Health Information Technology, School of Paramedical, and Rehabilitation Sciences, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Unit of Health Information Technology, Department of Health Management and Economy Sciences, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Development & Coordination Scientific Information, and Publications, Deputy of Research & Technology, Ministry of Health & Medical Education, Tehran, Iran. ,ghorbani@research.ac.ir
2- Unit of Health Information Technology, Department of Health Management and Economy Sciences, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Development & Coordination Scientific Information, and Publications, Deputy of Research & Technology, Ministry of Health & Medical Education, Tehran, Iran. ,
Full-Text [PDF 578 kb]
(426 Downloads)
| Abstract (HTML) (2615 Views)
Full-Text: (668 Views)
Introduction
One of the first medical interventions used to treat pain and suffering is medication; however, studies show that the medication itself can be harmful [1]. Unpleasant or harmful reactions that result from an intervention using a medical drug or an unwanted drug response that occurs at drug doses prescribed for treatment or for the regulation of physiological functions are referred to as adverse drug reactions (ADRs) [2].
ADRs can lead to patient hospitalization or prolonged hospitalization, which increases clinical costs. Therefore, ADRs impose a great burden on the healthcare system, which has a significant impact on the quality of life of patients. On the other hand, ADR increases morbidity and mortality worldwide. With the increasing complexity of drugs used to treat various diseases in different societies, this issue has become an important public health concern [3]. The importance of drug safety lies in all countries, particularly developing ones, as the occurrence of ADRs and other drug problems can provide valuable information for a region or country. Despite its high educational value, this information significantly impacts regulatory decisions at the national level [4]. Therefore, policymakers strive to develop the healthcare system by utilizing new technologies to prevent unwanted drug side effects in the future while ensuring effective medical care [5, 6, 7]. The first ideas for computerizing the registration and investigation of ADR were formed in the mid-1970s [8]. The international spontaneous reporting system on yellow cards is one of the most valuable methods of monitoring drug side effects and is of unique importance in identifying severe and rare drug reactions [9, 10]. In order to create a valid drug complication report, both the patient and the reporting person should provide biographical information, details of the unwanted drug side effects, and information about the drug [11].
In Iran, the status and efficiency of the pharmacovigilance system are unknown, and there is not enough information about its performance and effectiveness. Most of the research conducted focuses on the evaluation of knowledge, attitude, and performance regarding the pharmacovigilance system. Meanwhile, the existence of a drug monitoring system has led to improvements in the quality and quantity of drug side effect reports and has provided more accurate decision-making in this field [12]. Voluntary reporting systems have been used worldwide in the field of pharmacovigilance for half a century. Along with the activities carried out in many countries, and also in Iran, the National Center for Registration, and Investigation of ADRs is responsible for organizing the activities related to ADRs and for collecting and reviewing reports at the national level [13]. The Center for Registration and Investigation of Adverse Drugs, as the only national center in the country, collects and registers reports of drug side effects observed by the medical community to compile ADR reports [12]. For the successful implementation of systems such as an ADR reporting system, users of information systems should feel comfortable and satisfied while working with them. Therefore, designing a user interface for these systems should be based on a series of standard principles and rules. Usability tests usually refer to observing the user’s interaction with the system display (or user interface) while performing tasks [14]. Recent studies have shown that the insufficient pharmacovigilance system to monitor ADRs, along with the need for more awareness and knowledge about ADR reporting, has caused many problems globally. Severe ADRs lead to numerous medical and economic consequences. Therefore, it seems necessary to evaluate the ADR system at the first stage to avoid more harmful effects of prescribed drugs [15].
Different evaluation methods are available for systems. Exploratory evaluation is an efficient, easy, and low-cost method used to evaluate the usability of information systems. Using this method, a large number of usability problems can be identified in a limited period of time. Also, it is possible to conduct evaluations with a small number of evaluators without involving system users. This method was first recognized by Jacob Nilsson. Nielsen’s method uses principles to identify system usability problems and determine their severity [16].
Studies have shown that this method is an effective and efficient method for evaluating information systems in the field of health [17]. In this method, any violation of the ten principles in the design of the user interface of the system is identified as a usability problem by the evaluators. Errors can potentially create obstacles and limitations for the effective interaction of users with the system’s user interface. Finally, the evaluation results can be used to improve the user interface of the system [18]. Many studies have introduced heuristic evaluation as a successful method for assessing the usability of information systems in the health sector [19]. Also, many studies have evaluated the application of drug side effect reporting systems in some developed countries, such as Australia and the United States; however, the application of this system in some developing countries, such as Iran has not been sufficiently studied [20, 21].
Accordingly, this study aimed to evaluate the usability of the national ADR reporting system with the aim of identifying the challenges and priorities of the required pharmaceutical care. The results of this study can help a foundation for addressing these challenges and, consequently, promote health and safety in the use of medicines.
Methods
This descriptive and cross-sectional study was conducted in October 2022 on the national system for ADR reports, which was launched by the Food and Drug Organization of the Ministry of Health and Medical Education. In this study, the system for recording unwanted drug side effects used in 28 hospitals affiliated with Mashhad University of Medical Sciences was evaluated.
The system consists of four sections: Expert section, reports, reporter, and assessor. It can be accessed at Adverse Drug Reaction Reporting System [22]. The heuristic method was used to reveal the ADR system usability problems. A validated checklist based on Pierotti’s heuristic evaluation was used for data gathering. This checklist includes 13 principles of heuristic evaluation (Table 1) and contains 292 questions [23].

The usability assessment was performed by seven experts: One Ph.D. specialist in health information management with eight years of practical experience in health systems and ADR, three computer science experts with ten years of experience in health management systems, and three Ph.D. specialists in medical informatics with seven years of experience in evaluating information management systems. All usability evaluators had completed a course on heuristic evaluation, and to improve the quality of the evaluation, explanations were given to the evaluators to match the personal interpretations of the checklists. In this study, the evaluators examined the user interface of the system and independently evaluated its design compliance with the predefined principles. Any violations were noted as a usability issue. After completing the evaluation, the identified problems were discussed in a meeting. The list of problems and their frequency was specified for each issue. Each problem identified in the list was recognized by more than one evaluator and agreed upon by all evaluators. Any disagreements on identified problems were resolved through consensus.
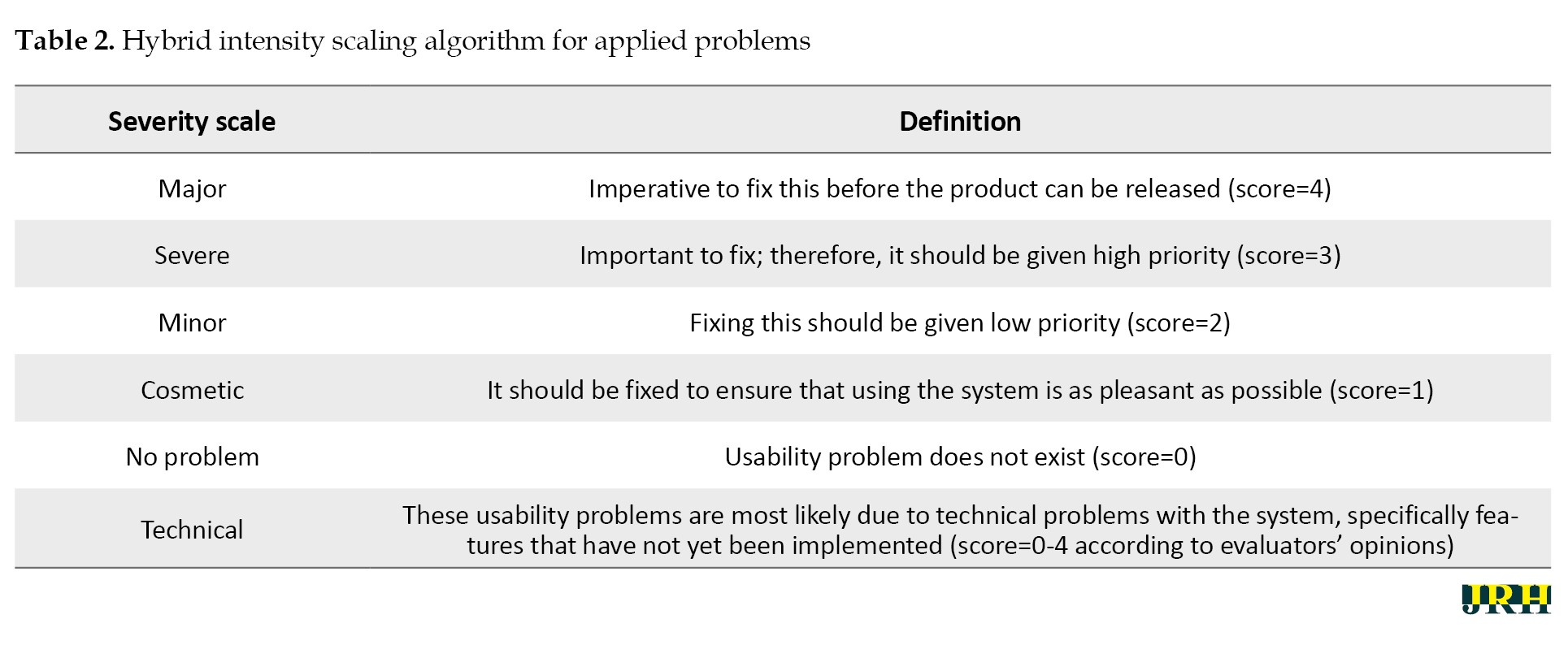
This study was conducted in two phases. First, to determine the severity of the problems, a combined method utilizing Nielsen’s severity rating scale and Tampere unit for computer-human interaction (TAUCHI) was used. According to the TAUCHI scale (Table 2), if a feature has not yet been implemented in the system, evaluators consider it a technical usability problem.
Therefore, usability problems were identified due to a lack of design or the absence of a feature in the system. Second, the mean severity of each usability problem was estimated.
Results
A total of 265 problems were identified in the usability of this system (Table 3).

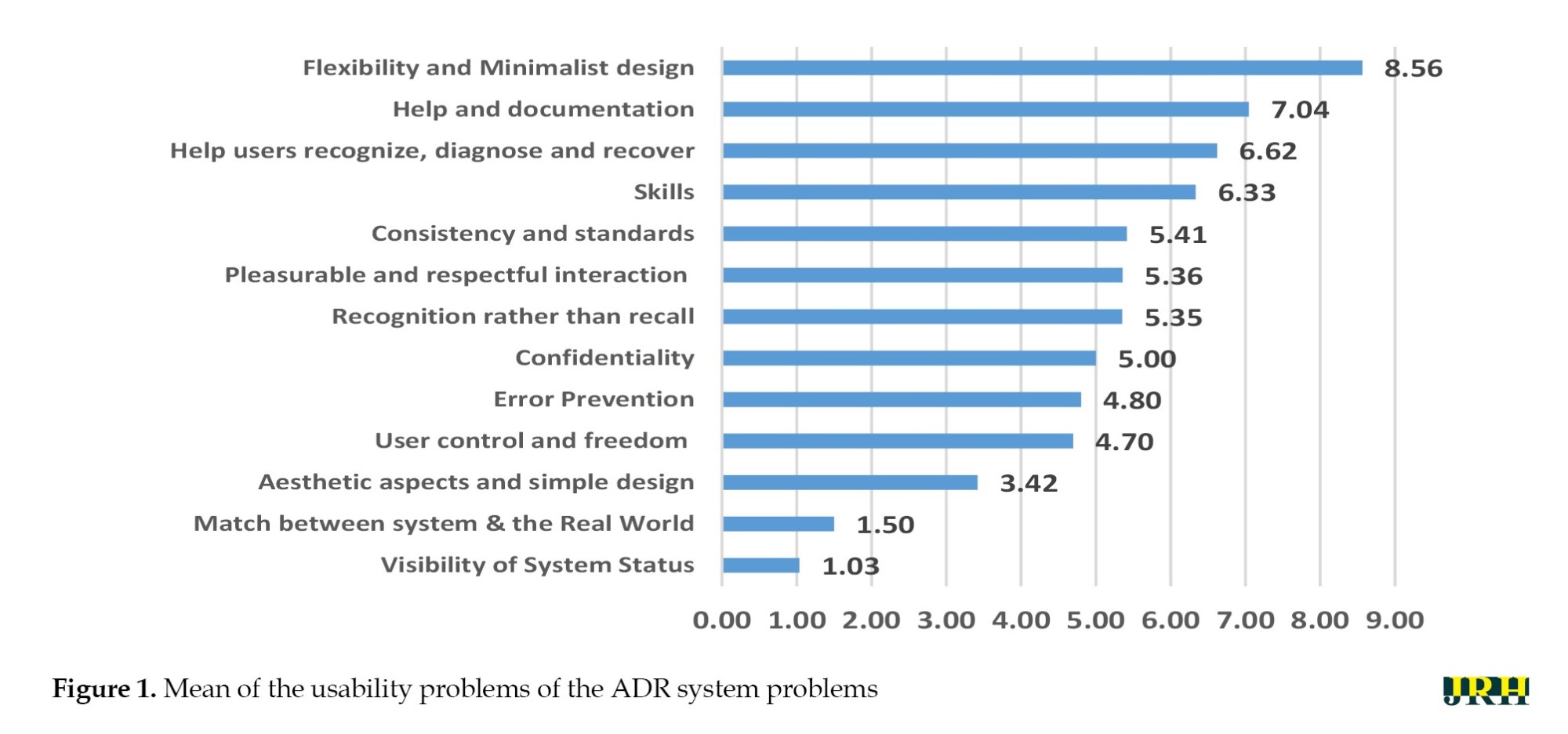
The severities of the identified problems, according to the defined criteria, are as follows: Severe problems 10(3.73%), minor problems 135(50.94%), repairable but not necessary 120(45.28%), and improvable but not necessary 120(45.28%). According to the evaluators, both major and technical problems were zero. The mean range of difficulty was between 1.03 and 8.56. The principle of “system flexibility” exhibited the highest level of severity for the problem (Figure 1). In the visibility of system status, the most mentioned problems were a lack of transparency in the system, which prevents users from understanding the state of the system and the possible ways to perform the desired action; failure to retain information exchanged between the system and the user during several consecutive responses in <2 seconds; a lack of clarity in the naming of graphical menus in the user interface and the terms used within them; and insufficient response time of the system relative to the cognitive processing speed of the user.
The most common problems identified between the system and the real world were active GUI menus that do not provide any functionality for the user; the system’s failure to automatically insert commas in numerical values greater than 9999, as well as not inserting the dollar sign; input data codes for cost values that are not meaningful; the system not automatically including spaces for decimal numbers; and the command language not allowing the use of both full names and abbreviations. The biggest issues regarding user control and freedom over the system were the inability to cancel changes at the level of activity, data entry, or a complete set of activities; restrictions on editing characters while entering commands; the inability to copy and modify existing data; difficulties in navigating between overlapping windows in the system; and a lack of options for personalizing the system for the user. The most significant problems related to compliance with uniformity and standards were the absence of prominent visual indicators to identify the active window in the system; the “exit” option in the menus not always being displayed at the end of the list; a lack of a specific and integrated location for online instructions on the screen; insufficient color-coded instructions; the command language for system commands lacking a uniform, natural, and memorable syntax; and the absence of consecutive page numbers when information is entered across several consecutive pages.
The most common problems related to helping users diagnose and recover from errors included: The absence of audio signals for error warnings and a lack of differentiation in error message details for expert and beginner users. The most significant issues related to error prevention were: The absence of default values for data input pages and dialog boxes, as well as the failure to display the number of character spaces in data input pages and dialog boxes.
In the recognition rather than recall category, the most problems mentioned included: The absence of an obvious distinction between single-choice and multi-choice menus to indicate the importance of different items on the screen; not using appropriate lines to demarcate components within a group; failure to use capital letters or different typographic styles (such as italics) to highlight the importance of various items on the screen; not maintaining the same color for similar items; and the failure to include default selections in the menu items.
Regarding system flexibility and minimalist design, the most frequently mentioned problems were the lack of different levels of error detail for advanced and novice users; insufficient access to beginner and advanced level instructions; the inability to define synonyms desired by the user for commands; and the absence of predefined shortcodes, such as SUM (similar to Excel), despite the system having type-ahead capability. Additionally, there was no option to define macros or hotkeys in nested boxes for skilled users. In terms of aesthetic aspects and minimalist design, the most commonly mentioned problems were the failure to use highlighted lines and plain areas to distinguish icons from one another more easily; not adhering to a hierarchical structure between the menus; and the lack of prominence and distinctness of each icon compared to the background screen.
Discussion
In this study, we applied a heuristic evaluation checklist as well as a hybrid severity scaling algorithm to evaluate the ADR system. The findings suggested many technical usability problems within the ADR system. A total of 265 problems were identified in the usability of this system. The principle of “system flexibility” exhibited the highest level of severity for the problems identified. “Help and instructions for use”, and “user assistance in error detection and recovery” were categorized as the second and third principles, respectively. Also, the principle of system clarity, with a severity score of 1.03, represented the lowest level of severity.
The axis of “observance of uniformity and standards” with 51 problems and an average intensity of 5.41 had the highest number of usability issues. In the second and third ranks, “detection instead of reminders” and “system clarity status” had 40 and 29 problems, respectively. Regarding confidentiality, only 3 problems were reported. The results of this study showed that the principles of “detection rather than reminders” and “uniformity and standards” had the most usability problems, respectively. The findings of studies by Nabovati et al. [24], Khajouei et al. [25], Rezaei-Hachesu et al. [26], and Ebnehoseini et al. [27] on a heuristic evaluation of HIS subsystems, as well as Klarich et al. on smart infusion pumps [28], revealed similar issues. This component indicates that users’ memory load should be minimized by clearly exposing objects, actions, and options.
Users should not need to remember system facilities [29]. In the studies mentioned, these principles were identified as having the most frequent usability problems, and new types of usability problems have emerged. The results of the usability evaluations conducted by Agharezaei et al. [30] and Ebnehoseini et al. [27] on subsystems of HIS and EHR also showed numerous usability problems related to the principle of “uniformity and standards”. The findings of this study confirmed these results. Also, another study indicated that access to standard data in an organized format is essential for providing proper and timely services. Designers wishing to develop or update an HIS should pay particular attention to the usability issues within these systems [31].
In the study by Agharezaei et al. on the examination of the laboratory information system, the highest number of problems was related to “flexibility and efficiency of use”, and the lowest was related to “assisting the user in error detection and recovery” [30]. Harrington et al.’s study of self-management software for diabetes in 2022, which employed Pareto analysis, revealed that 29(57%) of the exploratory violations were related to “system-real-world compliance,” and 8(16%) were linked to “aesthetic and simple design” [32]. The results of studies by Abedi and Khajouei [29] and Nabovati et al. [24] indicated that the component of “flexibility and compatibility of the system” had the highest number of problems, which is in line with the results of the present study. Another study identified that the mean severity score for flexibility and efficiency of use, based on heuristic evaluation of the clinical decision support system, was 0.66, with scores closer to zero indicating a more usable system [33]. In the study by Ahmadian et al. [34], the components of system adaptation to the real world and aesthetic and simple design aspects faced the most challenges in evaluating the radiology information system. In this system, guidance and instructions had the lowest reported problems, which is in line with the results of the present study.
In another study, challenges, and problems related to ADRs were pointed out. The most important challenges included a lack of knowledge to detect ADRs, time constraints, an insufficient information technology system, lack of support, getting stuck in routine practices, and not recognizing the importance of recording ADRs. Addressing these issues can improve the recording of ADRs and may enhance patient safety [35].
Therefore, paying attention to the drug side effect registration system is very important for patient safety. While solving the problems in the system, education and information about this system should be provided to the public and the staff of the healthcare system.
One of the strengths of this study is a professional assessor who has significant experience in working with information systems in the health domain and who could effectively diagnose system problems. The second strength is the use of this evaluation method as a simple and systematic approach to determine system usability problems at a specific point in time. The heuristic evaluation method revealed more problems with a lower average severity compared to other methods. Regarding the evaluation goal, this method was effective in improving the effectiveness and satisfaction of information systems in the health domain [36].
Also, the limitations of this study were the lack of full access to the mentioned system, which was contingent upon making the necessary arrangements with the Food and Drug Organization of the Ministry of Health and Medical Education.
Conclusion
Many health information systems in Iran, despite their widespread use in the country, have usability problems. This can affect the quality of user interaction with the system, and consequently, the outcomes of proper care. Therefore, it is necessary to evaluate the applicability of organizational information systems at each stage of their life cycle and during the development of those systems in order to improve their design. Finally, the evaluation of the system by experts should emphasize greater attention to reminders and warnings, as well as the clarity and flexibility of the system, to address and resolve the problems of the reporting system.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Research Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.REC.1398.056).
Funding
This work was supported by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No.: 971701).
Authors' contributions
Conceptualization: Marziyhe Meraji; Methodology: Marziyhe Meraji, Haniyeh Bameri, and Nahid Ramezanghorbani: Investigation: Zahra Ebnehoseini and Haniyeh Bameri: Writing the original draft: MarziyheMeraji, Nahid Ramezanghorbani, Haniyeh Bameri and Sanaz Sadat Mahmoodian; Review and editing: All authors.
Conflict of interest
The authors declared conflict of interest.
Acknowledgments
The authors would like to thank Mashhad University of Medical Sciences, Mashhad, Iran for supporting this project.
References
One of the first medical interventions used to treat pain and suffering is medication; however, studies show that the medication itself can be harmful [1]. Unpleasant or harmful reactions that result from an intervention using a medical drug or an unwanted drug response that occurs at drug doses prescribed for treatment or for the regulation of physiological functions are referred to as adverse drug reactions (ADRs) [2].
ADRs can lead to patient hospitalization or prolonged hospitalization, which increases clinical costs. Therefore, ADRs impose a great burden on the healthcare system, which has a significant impact on the quality of life of patients. On the other hand, ADR increases morbidity and mortality worldwide. With the increasing complexity of drugs used to treat various diseases in different societies, this issue has become an important public health concern [3]. The importance of drug safety lies in all countries, particularly developing ones, as the occurrence of ADRs and other drug problems can provide valuable information for a region or country. Despite its high educational value, this information significantly impacts regulatory decisions at the national level [4]. Therefore, policymakers strive to develop the healthcare system by utilizing new technologies to prevent unwanted drug side effects in the future while ensuring effective medical care [5, 6, 7]. The first ideas for computerizing the registration and investigation of ADR were formed in the mid-1970s [8]. The international spontaneous reporting system on yellow cards is one of the most valuable methods of monitoring drug side effects and is of unique importance in identifying severe and rare drug reactions [9, 10]. In order to create a valid drug complication report, both the patient and the reporting person should provide biographical information, details of the unwanted drug side effects, and information about the drug [11].
In Iran, the status and efficiency of the pharmacovigilance system are unknown, and there is not enough information about its performance and effectiveness. Most of the research conducted focuses on the evaluation of knowledge, attitude, and performance regarding the pharmacovigilance system. Meanwhile, the existence of a drug monitoring system has led to improvements in the quality and quantity of drug side effect reports and has provided more accurate decision-making in this field [12]. Voluntary reporting systems have been used worldwide in the field of pharmacovigilance for half a century. Along with the activities carried out in many countries, and also in Iran, the National Center for Registration, and Investigation of ADRs is responsible for organizing the activities related to ADRs and for collecting and reviewing reports at the national level [13]. The Center for Registration and Investigation of Adverse Drugs, as the only national center in the country, collects and registers reports of drug side effects observed by the medical community to compile ADR reports [12]. For the successful implementation of systems such as an ADR reporting system, users of information systems should feel comfortable and satisfied while working with them. Therefore, designing a user interface for these systems should be based on a series of standard principles and rules. Usability tests usually refer to observing the user’s interaction with the system display (or user interface) while performing tasks [14]. Recent studies have shown that the insufficient pharmacovigilance system to monitor ADRs, along with the need for more awareness and knowledge about ADR reporting, has caused many problems globally. Severe ADRs lead to numerous medical and economic consequences. Therefore, it seems necessary to evaluate the ADR system at the first stage to avoid more harmful effects of prescribed drugs [15].
Different evaluation methods are available for systems. Exploratory evaluation is an efficient, easy, and low-cost method used to evaluate the usability of information systems. Using this method, a large number of usability problems can be identified in a limited period of time. Also, it is possible to conduct evaluations with a small number of evaluators without involving system users. This method was first recognized by Jacob Nilsson. Nielsen’s method uses principles to identify system usability problems and determine their severity [16].
Studies have shown that this method is an effective and efficient method for evaluating information systems in the field of health [17]. In this method, any violation of the ten principles in the design of the user interface of the system is identified as a usability problem by the evaluators. Errors can potentially create obstacles and limitations for the effective interaction of users with the system’s user interface. Finally, the evaluation results can be used to improve the user interface of the system [18]. Many studies have introduced heuristic evaluation as a successful method for assessing the usability of information systems in the health sector [19]. Also, many studies have evaluated the application of drug side effect reporting systems in some developed countries, such as Australia and the United States; however, the application of this system in some developing countries, such as Iran has not been sufficiently studied [20, 21].
Accordingly, this study aimed to evaluate the usability of the national ADR reporting system with the aim of identifying the challenges and priorities of the required pharmaceutical care. The results of this study can help a foundation for addressing these challenges and, consequently, promote health and safety in the use of medicines.
Methods
This descriptive and cross-sectional study was conducted in October 2022 on the national system for ADR reports, which was launched by the Food and Drug Organization of the Ministry of Health and Medical Education. In this study, the system for recording unwanted drug side effects used in 28 hospitals affiliated with Mashhad University of Medical Sciences was evaluated.
The system consists of four sections: Expert section, reports, reporter, and assessor. It can be accessed at Adverse Drug Reaction Reporting System [22]. The heuristic method was used to reveal the ADR system usability problems. A validated checklist based on Pierotti’s heuristic evaluation was used for data gathering. This checklist includes 13 principles of heuristic evaluation (Table 1) and contains 292 questions [23].

The usability assessment was performed by seven experts: One Ph.D. specialist in health information management with eight years of practical experience in health systems and ADR, three computer science experts with ten years of experience in health management systems, and three Ph.D. specialists in medical informatics with seven years of experience in evaluating information management systems. All usability evaluators had completed a course on heuristic evaluation, and to improve the quality of the evaluation, explanations were given to the evaluators to match the personal interpretations of the checklists. In this study, the evaluators examined the user interface of the system and independently evaluated its design compliance with the predefined principles. Any violations were noted as a usability issue. After completing the evaluation, the identified problems were discussed in a meeting. The list of problems and their frequency was specified for each issue. Each problem identified in the list was recognized by more than one evaluator and agreed upon by all evaluators. Any disagreements on identified problems were resolved through consensus.
This study was conducted in two phases. First, to determine the severity of the problems, a combined method utilizing Nielsen’s severity rating scale and Tampere unit for computer-human interaction (TAUCHI) was used. According to the TAUCHI scale (Table 2), if a feature has not yet been implemented in the system, evaluators consider it a technical usability problem.

Therefore, usability problems were identified due to a lack of design or the absence of a feature in the system. Second, the mean severity of each usability problem was estimated.
Results
A total of 265 problems were identified in the usability of this system (Table 3).

The severities of the identified problems, according to the defined criteria, are as follows: Severe problems 10(3.73%), minor problems 135(50.94%), repairable but not necessary 120(45.28%), and improvable but not necessary 120(45.28%). According to the evaluators, both major and technical problems were zero. The mean range of difficulty was between 1.03 and 8.56. The principle of “system flexibility” exhibited the highest level of severity for the problem (Figure 1). In the visibility of system status, the most mentioned problems were a lack of transparency in the system, which prevents users from understanding the state of the system and the possible ways to perform the desired action; failure to retain information exchanged between the system and the user during several consecutive responses in <2 seconds; a lack of clarity in the naming of graphical menus in the user interface and the terms used within them; and insufficient response time of the system relative to the cognitive processing speed of the user.
The most common problems identified between the system and the real world were active GUI menus that do not provide any functionality for the user; the system’s failure to automatically insert commas in numerical values greater than 9999, as well as not inserting the dollar sign; input data codes for cost values that are not meaningful; the system not automatically including spaces for decimal numbers; and the command language not allowing the use of both full names and abbreviations. The biggest issues regarding user control and freedom over the system were the inability to cancel changes at the level of activity, data entry, or a complete set of activities; restrictions on editing characters while entering commands; the inability to copy and modify existing data; difficulties in navigating between overlapping windows in the system; and a lack of options for personalizing the system for the user. The most significant problems related to compliance with uniformity and standards were the absence of prominent visual indicators to identify the active window in the system; the “exit” option in the menus not always being displayed at the end of the list; a lack of a specific and integrated location for online instructions on the screen; insufficient color-coded instructions; the command language for system commands lacking a uniform, natural, and memorable syntax; and the absence of consecutive page numbers when information is entered across several consecutive pages.
The most common problems related to helping users diagnose and recover from errors included: The absence of audio signals for error warnings and a lack of differentiation in error message details for expert and beginner users. The most significant issues related to error prevention were: The absence of default values for data input pages and dialog boxes, as well as the failure to display the number of character spaces in data input pages and dialog boxes.
In the recognition rather than recall category, the most problems mentioned included: The absence of an obvious distinction between single-choice and multi-choice menus to indicate the importance of different items on the screen; not using appropriate lines to demarcate components within a group; failure to use capital letters or different typographic styles (such as italics) to highlight the importance of various items on the screen; not maintaining the same color for similar items; and the failure to include default selections in the menu items.
Regarding system flexibility and minimalist design, the most frequently mentioned problems were the lack of different levels of error detail for advanced and novice users; insufficient access to beginner and advanced level instructions; the inability to define synonyms desired by the user for commands; and the absence of predefined shortcodes, such as SUM (similar to Excel), despite the system having type-ahead capability. Additionally, there was no option to define macros or hotkeys in nested boxes for skilled users. In terms of aesthetic aspects and minimalist design, the most commonly mentioned problems were the failure to use highlighted lines and plain areas to distinguish icons from one another more easily; not adhering to a hierarchical structure between the menus; and the lack of prominence and distinctness of each icon compared to the background screen.
Discussion
In this study, we applied a heuristic evaluation checklist as well as a hybrid severity scaling algorithm to evaluate the ADR system. The findings suggested many technical usability problems within the ADR system. A total of 265 problems were identified in the usability of this system. The principle of “system flexibility” exhibited the highest level of severity for the problems identified. “Help and instructions for use”, and “user assistance in error detection and recovery” were categorized as the second and third principles, respectively. Also, the principle of system clarity, with a severity score of 1.03, represented the lowest level of severity.
The axis of “observance of uniformity and standards” with 51 problems and an average intensity of 5.41 had the highest number of usability issues. In the second and third ranks, “detection instead of reminders” and “system clarity status” had 40 and 29 problems, respectively. Regarding confidentiality, only 3 problems were reported. The results of this study showed that the principles of “detection rather than reminders” and “uniformity and standards” had the most usability problems, respectively. The findings of studies by Nabovati et al. [24], Khajouei et al. [25], Rezaei-Hachesu et al. [26], and Ebnehoseini et al. [27] on a heuristic evaluation of HIS subsystems, as well as Klarich et al. on smart infusion pumps [28], revealed similar issues. This component indicates that users’ memory load should be minimized by clearly exposing objects, actions, and options.
Users should not need to remember system facilities [29]. In the studies mentioned, these principles were identified as having the most frequent usability problems, and new types of usability problems have emerged. The results of the usability evaluations conducted by Agharezaei et al. [30] and Ebnehoseini et al. [27] on subsystems of HIS and EHR also showed numerous usability problems related to the principle of “uniformity and standards”. The findings of this study confirmed these results. Also, another study indicated that access to standard data in an organized format is essential for providing proper and timely services. Designers wishing to develop or update an HIS should pay particular attention to the usability issues within these systems [31].
In the study by Agharezaei et al. on the examination of the laboratory information system, the highest number of problems was related to “flexibility and efficiency of use”, and the lowest was related to “assisting the user in error detection and recovery” [30]. Harrington et al.’s study of self-management software for diabetes in 2022, which employed Pareto analysis, revealed that 29(57%) of the exploratory violations were related to “system-real-world compliance,” and 8(16%) were linked to “aesthetic and simple design” [32]. The results of studies by Abedi and Khajouei [29] and Nabovati et al. [24] indicated that the component of “flexibility and compatibility of the system” had the highest number of problems, which is in line with the results of the present study. Another study identified that the mean severity score for flexibility and efficiency of use, based on heuristic evaluation of the clinical decision support system, was 0.66, with scores closer to zero indicating a more usable system [33]. In the study by Ahmadian et al. [34], the components of system adaptation to the real world and aesthetic and simple design aspects faced the most challenges in evaluating the radiology information system. In this system, guidance and instructions had the lowest reported problems, which is in line with the results of the present study.
In another study, challenges, and problems related to ADRs were pointed out. The most important challenges included a lack of knowledge to detect ADRs, time constraints, an insufficient information technology system, lack of support, getting stuck in routine practices, and not recognizing the importance of recording ADRs. Addressing these issues can improve the recording of ADRs and may enhance patient safety [35].
Therefore, paying attention to the drug side effect registration system is very important for patient safety. While solving the problems in the system, education and information about this system should be provided to the public and the staff of the healthcare system.
One of the strengths of this study is a professional assessor who has significant experience in working with information systems in the health domain and who could effectively diagnose system problems. The second strength is the use of this evaluation method as a simple and systematic approach to determine system usability problems at a specific point in time. The heuristic evaluation method revealed more problems with a lower average severity compared to other methods. Regarding the evaluation goal, this method was effective in improving the effectiveness and satisfaction of information systems in the health domain [36].
Also, the limitations of this study were the lack of full access to the mentioned system, which was contingent upon making the necessary arrangements with the Food and Drug Organization of the Ministry of Health and Medical Education.
Conclusion
Many health information systems in Iran, despite their widespread use in the country, have usability problems. This can affect the quality of user interaction with the system, and consequently, the outcomes of proper care. Therefore, it is necessary to evaluate the applicability of organizational information systems at each stage of their life cycle and during the development of those systems in order to improve their design. Finally, the evaluation of the system by experts should emphasize greater attention to reminders and warnings, as well as the clarity and flexibility of the system, to address and resolve the problems of the reporting system.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Research Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.REC.1398.056).
Funding
This work was supported by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No.: 971701).
Authors' contributions
Conceptualization: Marziyhe Meraji; Methodology: Marziyhe Meraji, Haniyeh Bameri, and Nahid Ramezanghorbani: Investigation: Zahra Ebnehoseini and Haniyeh Bameri: Writing the original draft: MarziyheMeraji, Nahid Ramezanghorbani, Haniyeh Bameri and Sanaz Sadat Mahmoodian; Review and editing: All authors.
Conflict of interest
The authors declared conflict of interest.
Acknowledgments
The authors would like to thank Mashhad University of Medical Sciences, Mashhad, Iran for supporting this project.
References
- Lockhart KL, Keil FC. I. Introduction: Understanding medicines and medical interventions. Monographs of the Society for Research in Child Development. 2018; 83(2):7-32. [DOI:10.1111/mono.12361] [PMID] [PMCID]
- Coleman JJ, Pontefract SK. Adverse drug reactions. Clinical Medicine. 2016; 16(5):481-5. [DOI:10.7861/clinmedicine.16-5-481] [PMID] [PMCID]
- Khalil H, Huang C. Adverse drug reactions in primary care: A scoping review. BMC Health Services Research. 2020; 20(1):5. [DOI:10.1186/s12913-019-4651-7] [PMID] [PMCID]
- Keche Y, Gaikwad N, Dhaneria S. Preventability, predictability, severity and causality assessment of adverse drug reactions reported from a teaching hospital in chhattisgarh: A retrospective analysis. Journal of Family Medicine and Primary Care. 2021; 10(7):2541-5. [DOI:10.4103/jfmpc.jfmpc_2374_20] [PMID] [PMCID]
- Dalhoff K, Andersen JT, Jimenez-Solem E, Dalhoff KP. A new beginning: Adverse drug reaction manager: A way of increasing the number of spontaneous reporting. Adverse Drug Reaction Bulletin. 2018; 311(1):1203-6. [DOI:10.1097/FAD.0000000000000035]
- IRM A. Data analytics in medicine: Concepts, methodologies, tools and applications. Hershey: IGI Global; 2020. [Link]
- Khan N. Mobile health technology to enhance healthcare service delivery in developing nations (Saudi Arabia) [doctoral thesis]. Orlando: University of Central Florida; 2020. [Link]
- Meraji M, Fazaeli S, Ebnehoseini Z, Bameri H. [Reporting adverse drug reactions with emphasis on the designing national minimum data set (Persian). Journal of Modern Medical Information Sciences. 2022; 8(1):62-3. [Link]
- Al Dweik R, Stacey D, Kohen D, Yaya S. Factors affecting patient reporting of adverse drug reactions: A systematic review. British Journal of Clinical Pharmacology. 2017; 83(4):875-83. [DOI:10.1111/bcp.13159] [PMID] [PMCID]
- Moayeri A, Aminshokravi F, Tavafian S, Moayeri A. [Assessing related factors on the illicit use of medications in Abbas Abad City (mazandaran): A cross sectional study (Persian)]. Journal of Ilam University of Medical Sciences. 2014; 22(5):11-9. [Link]
- Guideline IHT. Post-approval safety data management: definitions and standards for expedited reporting E2D. Paper presented at: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals For Human Use. 12 November 2003; Madrid, Spain. [Link]
- Khalili M, Sharifi H, Mesgarpour B, Kheirandish M, Olsson S, Javidnikou N, et al. Evaluation of pharmacovigilance system in Iran. International Journal of Health Policy and Management. 2022; 11(7):990-1000. [DOI:10.34172/ijhpm.2020.243] [PMID]
- Shalviri G, Mohammad K, Majdzadeh S, Gholami K. [Comparing epidemiological methods in detecting drug safety signal in Iran (Persian)]. Iranian Journal of Epidemiology. 2005; 1(1 and 2):17-26. [Link]
- Parhizi R, Asgari A, Ayati M. The evaluation of the factors that affecting usage of the golestan system in the University of Birjand. International Journal of Research in Organizational Behavior and Human Resource Management. 2014; 2(4):213-23. [Link]
- Shukla S, Sharma P, Gupta P, Pandey S, Agrawal R, Rathour D, et al. Current scenario and future prospects of adverse drug reactions (ADRs) monitoring and reporting mechanisms in the rural areas of India. Current Drug Safety. 2024; 19(2):172-90. [DOI:10.2174/1574886318666230428144120] [PMID]
- Gonzalez-Holland E, Whitmer D, Moralez L, Mouloua M. Examination of the use of Nielsen’s 10 usability heuristics & outlooks for the future. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. 2017; 61(1):1472-5. [DOI:10.1177/1541931213601853]
- Tang Z, Johnson TR, Tindall RD, Zhang J. Applying heuristic evaluation to improve the usability of a telemedicine system. Telemedicine Journal and e-Health. 2006; 12(1):24-34. [DOI:10.1089/tmj.2006.12.24] [PMID]
- Rosson MB, Carroll JM. Scenario-based development of human-computer interaction In: Rosson MB, Carroll JM, editors. Usability engineering. San Francisco: Academic press; 2002. [DOI:10.1016/B978-155860712-5/50002-3]
- Joshi A, Arora M, Dai L, Price K, Vizer L, Sears A. Usability of a patient education and motivation tool using heuristic evaluation. Journal of Medical Internet Research. 2009; 11(4):e47. [DOI:10.2196/jmir.1244] [PMID] [PMCID]
- Ribeiro-Vaz I, Silva AM, Costa Santos C, Cruz-Correia R. How to promote adverse drug reaction reports using information systems - A systematic review and meta-analysis. BMC Medical Informatics and Decision Making. 2016; 16:27. [DOI:10.1186/s12911-016-0265-8] [PMID] [PMCID]
- Fossouo Tagne J, Yakob RA, Mcdonald R, Wickramasinghe N. A web-based tool to report adverse drug reactions by community pharmacists in Australia: Usability testing study. JMir Formative Research. 2023; 7:e48976. [DOI:10.2196/48976] [PMID] [PMCID]
- Xerox Corporation. Usability techniques: heuristic evaluation a system checklist [Internet]. 1998. [Updated 2025 February 3]. Available from: [Link]
- Islamic Republic of Iran 'Food and Drug Administration. Adverse drug reaction reporting system [Internet]. 2024. [Updated 2025 February 3]. Available from: [Link]
- Nabovati E, Vakili-Arki H, Eslami S, Khajouei R. Usability evaluation of laboratory and radiology information systems integrated into a hospital information system. Journal of Medical Systems. 2014; 38(4):35. [DOI:10.1007/s10916-014-0035-z] [PMID]
- Khajouei R, Azizi AA, Atashi A. [Atashi, Usability evaluation of an emergency information system: A heuristic evaluation (Persian)]. Journal of Health Administration. 2013; 16(51):61-72. [Link]
- Rezaei-Hachesu P, Pesianian E, Mohammadian M. Evaluating usability of radiology information systems in hospitals of Tabriz University of Medical Sciences. Acta Informatica Medica. 2016; 24(1):42-6. [DOI:10.5455/aim.2016.24.42-46] [PMID] [PMCID]
- Ebnehoseini Z, Tara M, Meraji M, Deldar K, Khoshronezhad F, Khoshronezhad S. Usability evaluation of an admission, discharge, and transfer information system: A heuristic evaluation. Open Access Macedonian Journal of Medical Sciences. 2018; 6(11):1941-5. [DOI:10.3889/oamjms.2018.392] [PMID] [PMCID]
- Klarich A, Noonan TZ, Reichlen C, Barbara SMJ, Cullen L, Pennathur PR. Usability of smart infusion pumps: A heuristic evaluation. Applied Ergonomics. 2022; 98:103584. [DOI:10.1016/j.apergo.2021.103584] [PMID]
- Khajouei R. [Evaluating the users' interaction problems with physiotherapy information system (Persian)]. Journal of Hospital. 2015; 14(3):83-92. [Link]
- Agharezaei Z, Khajouei R, Ahmadian L, Agharezaei L. [Usability evaluation of a laboratory information system (Persian)]. Director General. 2013; 10(2):1-2. [Link]
- McDonald CJ, Humphreys BL. The U.S. National Library of medicine and standards for electronic health records: One thing led to another. Information Services & Use. 2022; 42(1):81-94. [DOI:10.3233/ISU-210142] [PMID] [PMCID]
- Harrington L, Parker C, Ulanday K, Harrington C. Heuristic evaluation of a top-rated diabetes self-management app. Applied Clinical Informatics. 2021; 12(5):1014-20. [DOI:10.1055/s-0041-1736628] [PMID] [PMCID]
- Cho H, Keenan G, Madandola OO, Dos Santos FC, Macieira TGR, Bjarnadottir RI, Priola KJB, et al. Assessing the usability of a clinical decision support system: Heuristic evaluation. JMIR Human Factors. 2022; 9(2):e31758. [DOI:10.2196/31758] [PMID] [PMCID]
- Ahmadian L, Salehi F, Abedinzadeh A, Khatibi F. Usability evaluation of a radiology information system. Journal of Health Administration. 20(69):67-77. [Link]
- Geeven IPAC, Jessurun NT, Wasylewicz ATM, Drent M, Spuls PI, Hoentjen F, et al. Barriers and facilitators for systematically registering adverse drug reactions in electronic health records: A qualitative study with Dutch healthcare professionals. Expert Opinion on Drug Safety. 2022; 21(5):699-706. [DOI:10.1080/14740338.2022.2020756] [PMID]
- Price M, Bellwood P, Kitson N, Davies I, Weber J, Lau F. Conditions potentially sensitive to a personal health record (PHR) intervention, A systematic review. BMC Medical Informatics and Decision Making. 2015; 15:32. [DOI:10.1186/s12911-015-0159-1] [PMID] [PMCID]
Type of Study: Orginal Article |
Subject:
● Health Systems
Received: 2023/12/16 | Accepted: 2024/09/17 | Published: 2025/05/30
Received: 2023/12/16 | Accepted: 2024/09/17 | Published: 2025/05/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |