Volume 15, Issue 6 (Nov & Dec 2025)
J Research Health 2025, 15(6): 615-624 |
Back to browse issues page
Ethics code: Not applicable
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Paramitha N F, Awatara P I D, Soegiri Y J R. Association Between Obesity, Overweight, and Developing Long COVID: A Meta-analysis. J Research Health 2025; 15 (6) :615-624
URL: http://jrh.gmu.ac.ir/article-1-2620-en.html
URL: http://jrh.gmu.ac.ir/article-1-2620-en.html
1- Department of Pulmonology and Respiratory Medicine, Faculty of Medicine, dr. Saiful Anwar General Hospital, Universitas Brawijaya, Malang, Indonesia. , nfparamitha@gmail.com
2- Department of Internal Medicine, Faculty of Medicine, dr. Saiful Anwar General Hospital, Universitas Brawijaya, Malang, Indonesia.
3- Division of Infection Pulmonology, Department of Pulmonology and Respiratory Medicine, Faculty of Medicine, dr. Saiful Anwar General Hospital, Universitas Brawijaya, Malang, Indonesia.
2- Department of Internal Medicine, Faculty of Medicine, dr. Saiful Anwar General Hospital, Universitas Brawijaya, Malang, Indonesia.
3- Division of Infection Pulmonology, Department of Pulmonology and Respiratory Medicine, Faculty of Medicine, dr. Saiful Anwar General Hospital, Universitas Brawijaya, Malang, Indonesia.
Full-Text [PDF 696 kb]
(206 Downloads)
| Abstract (HTML) (2010 Views)
Full-Text: (351 Views)
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus, first identified in Wuhan, China, in late 2019. This disease presents various symptoms, including fever, cough, fatigue, shortness of breath, anosmia, and dysgeusia. In severe cases, it can progress to pneumonia, kidney and liver dysfunction, and even death [1]. The morbidity burden of the COVID-19 pandemic is becoming increasingly apparent and concerning. Long COVID, also referred to as post-COVID-19 condition, is broadly characterized by the persistence of symptoms for three months or longer following the acute phase of COVID-19 infection [2]. Furthermore, long COVID has been defined as the persistence of symptoms, or the development of new symptoms, relating to SARS-CoV-2 infection late during COVID-19, occurring at least 28 days after diagnosis [3, 4].
In the early stages of the COVID-19 pandemic, many individuals experiencing long COVID faced challenges, such as being undiagnosed, dismissed, inadequately evaluated, or improperly treated. This phenomenon was often described by affected individuals as “medical gaslighting.” The term “long COVID” was first introduced by patients on May 20, 2020, to represent the prolonged sequelae and chronic complications associated with COVID-19 infection [5]. The prevalence of long-term COVID-19 is still uncertain, but evidence is emerging that it is relatively common [6]. Studies around the world have reported various incidence rates for long COVID with different follow-up examination times after the acute infection, including 76% of people at 6 months [7], 32.6% at 60 days [8], 87% at 60 days [9], and 96% at 90 days [10]. Data from the UK’s Office for National Statistics (ONS), based on a nationally representative non-institutionalized sample of lab-confirmed COVID-19 cases, including asymptomatic ones, estimated a prevalence of 11.7% at 12 weeks post-testing positive, increasing to 17.7% when considering only those symptomatic during the acute phase of the illness [6, 11].
Long COVID has been associated with a broad range of symptoms and health impacts [12-15]. Several systematic reviews have shown the most prevalent symptoms to be fatigue, shortness of breath, muscle pain, joint pain, headache, cough, chest pain, altered smell, altered taste, and diarrhea [15-18]. Long COVID predominantly affected individuals aged 35-65 years, with males representing the majority of cases [5]. Individuals with certain medical conditions, such as type 2 diabetes, allergies, a history of post-viral fatigue, asthma, chronic lung disease, heart failure, and chronic kidney disease, as well as those experiencing a more severe acute COVID-19 illness, with a high body mass index (BMI), or being unvaccinated, are at greater risk of developing long COVID. These groups are also more likely to experience severe and persistent symptoms [10]. Another meta-analysis identified overweight and obesity as potential risk factors for SARS-CoV-2 infection. However, this association was not consistently supported by serological data [19].
In the Lancet respiratory medicine, the PHOSP-COVID Collaborative Group reported the latest results from the UK-based, multicentre, prospective post-hospitalization COVID-19 (PHOSP-COVID) study, in which the investigators identified systemic inflammation and obesity as factors that might be associated with long COVID, representing potentially treatable traits in people with more severe post-COVID-19 symptoms [20]. The mechanisms underlying the long-term persistence of symptoms are unknown. A potential hypothesis is that the hyperinflammation associated with acute COVID-19 leads to a persistent inflammatory state following COVID-19, associated with dysregulated immunity and multiorgan dysfunction. Obesity also leads to local and systemic chronic inflammation, which in turn results in various chronic immune and metabolic diseases, such as type 2 diabetes, cancer, atherosclerosis, and inflammatory bowel disease. It can be hypothesized that obesity and obesity-related comorbidities may adversely affect the recovery of discharged patients with COVID-19 [20, 21]. Due to the evolving clinical spectrum of COVID-19, which is influenced by differences in variants, vaccines, and susceptibility by ethnicity and race, it is extremely challenging to define post-COVID-19 syndromes [22].
There are indications that metabolic dysfunction may promote or enhance these syndromes [22]. Identifying and understanding modifiable determinants associated with manifestations of long COVID, including obesity/overweight, would help adapt treatment pathways for particular phenotypes. Overweight and obesity are defined as abnormal or excessive fat accumulation that presents a health risk. A BMI of over 25-29.9 is considered overweight, while a BMI over 30 is classified as obese [23]. Nonetheless, collecting the best evidence regarding the temporal aspects of this post-viral syndrome and designing the most appropriate treatment approaches can be achieved. The direct implications of COVID-19 on health and well-being are well-discussed elsewhere; what remains to be seen is whether this pandemic is exacerbating the growing obesity pandemic. A systematic review and meta-analysis by Bakaloudi et al. suggest an overall global trend of weight gain during the first COVID-19 lockdown [24]. To date, no studies have assessed the indirect impact of the COVID-19 pandemic on obesity and overweight risk factors that could explain this trend. Therefore, this paper managed to fill this gap by describing the effects of the COVID-19 pandemic and the needed countermeasures on obesity risk factors to explore the underlying mechanisms of the general trend of weight gain during the COVID-19 pandemic. In this study, the association between obesity, overweight, and long COVID in COVID-19 survivors was investigated.
Methods
Research design
This meta-analysis, conducted from March 2022 to August 2022, investigated the association between obese and overweight populations and the incidence of long-term COVID-19 in COVID-19 survivors. Preferred reporting items for systematic review and meta-analysis (PRISMA) were applied as the framework of this study [25].
Eligibility criteria
All data were obtained from articles published in PubMed, PMC, ScienceDirect, and Taylor and Francis. Potential outcomes were thoroughly identified, and a further search was conducted to obtain the papers that might be included in this study. The keywords were as follows: [“Obesity” or “overweight”] and [“long Covid” or “post-acute COVID-19 syndrome”] [26–28]. Two independent researchers (Nuansa Firgie Paramitha and Putu Ijiya Danta Awatara) extracted data using a pilot form, and further discussion was held if there was any disagreement. The articles included in this study were those that met the following inclusion criteria:
Population: Studies involving human participants aged 19 years or older, as specified in the study by Lee & Yoo [29].
Exposure: Studies must report pre-pandemic and post-pandemic BMI values or clearly define overweight (BMI 23–24.9 kg/m²) and obesity (BMI ≥25 kg/m²), consistent with the guidelines of the Asia-Pacific World Health Organization (WHO) or the Korean Society for the Study of Obesity (KSSO) referenced in the study.
Outcome: Studies assessing long COVID outcomes, defined as symptoms persisting for ≥12 weeks after acute COVID-19 infection, in line with WHO definitions.
Study type: Observational studies (cross-sectional, case-control, and cohort), either prospective or retrospective, that include effect size estimates (e.g. odd ratio, risk ratio) or data that allow calculation of the association between BMI status and long COVID development.
Publication date: Articles published between January 2020 and December 2024, to reflect the COVID-19 pandemic period covered in the referenced study.
Language: Articles must be published in English.
Availability: Full-text access must be available for data extraction.
Methodological quality: Studies must have moderate to high methodological quality, as assessed by the Newcastle-Ottawa scale (NOS), with a score of 5 or higher. This assessment focused on the rigor of the study design, including participant selection, comparability of groups, and outcome/exposure ascertainment.
Papers were excluded based on the following criteria:
Pediatric populations: Studies that exclusively involved participants under 19 years of age.
No long COVID data: Studies that did not measure or define long COVID or persistent symptoms post-infection.
No BMI classification: Studies that did not include clear definitions or measures of overweight or obesity.
Interventional trials or pharmacological studies: These were excluded unless baseline BMI and long COVID data were explicitly reported and analyzable.
Case reports, reviews, editorials, and conference abstracts: Non-original data or studies without full datasets were excluded.
Non-human or animal studies: Only studies involving human subjects were included.
High risk of bias: Studies assessed to have a high risk of bias using standardized tools (NOS) were excluded from quantitative synthesis.
Duplicate publications: In cases of multiple reports from the same dataset, only the most comprehensive or recent version was included.
Assessment of the article quality
Before the analysis process, the quality of the papers was assessed using the NOS. In this assessment, the evaluation of patient selection, group comparisons, and exposure assessment was performed. A score of less than 4 indicated that the paper had low quality, while a score of 5-6 indicated moderate quality. Papers with a score greater than or equal to 7 were considered high quality [30]. Articles included in this analysis were those of moderate to high quality. Two independent investigators (Nuansa Firgie Paramitha and Putu Ijiya Danta Awatara) conducted the study assessment using a pilot form. Other investigators (Putu Ijiya Danta Awatara) provided consultation if a disagreement arose.
Study measures
The predictor variable in this study was the obese and overweight populations among COVID-19 survivors. COVID-19 survivors are individuals who tested positive for the coronavirus and/or were later confirmed to have had the virus by testing positive for antibodies [31]. Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. BMI is a simple weight-for-height index commonly used to classify overweight and obesity in adults. It is defined as a person’s weight in kilograms divided by the square of his/her height in meters (kg/m2). For adults, the WHO defines overweight and obesity as follows: Underweight is a BMI less than 18.5, norm weight is a BMI between 18.5 and 24.9, overweight is a BMI greater than or equal to 25-29.9, and obesity is a BMI greater than or equal to 30 [32].
Statistical analysis
The comparison of obese and overweight populations in COVID-19 survivors was performed using a Z test, and their effects on the incidence of long COVID were determined by the calculation of the odds ratio and 95% confidence interval (CI). The data with potential bias and heterogeneity across the studies were assessed first before evaluating the correlation and effect estimates. Publication bias was analyzed using an Egger test, with the potential for publication bias considered if the P<0.05. Furthermore, heterogeneity among studies was assessed using a Q test, with data considered heterogeneous if the P<0.10. The calculation for our meta-analysis used Comprehensive Meta-Analysis software, version 3 (CMA, Chicago, US).
Results
Eligible studies
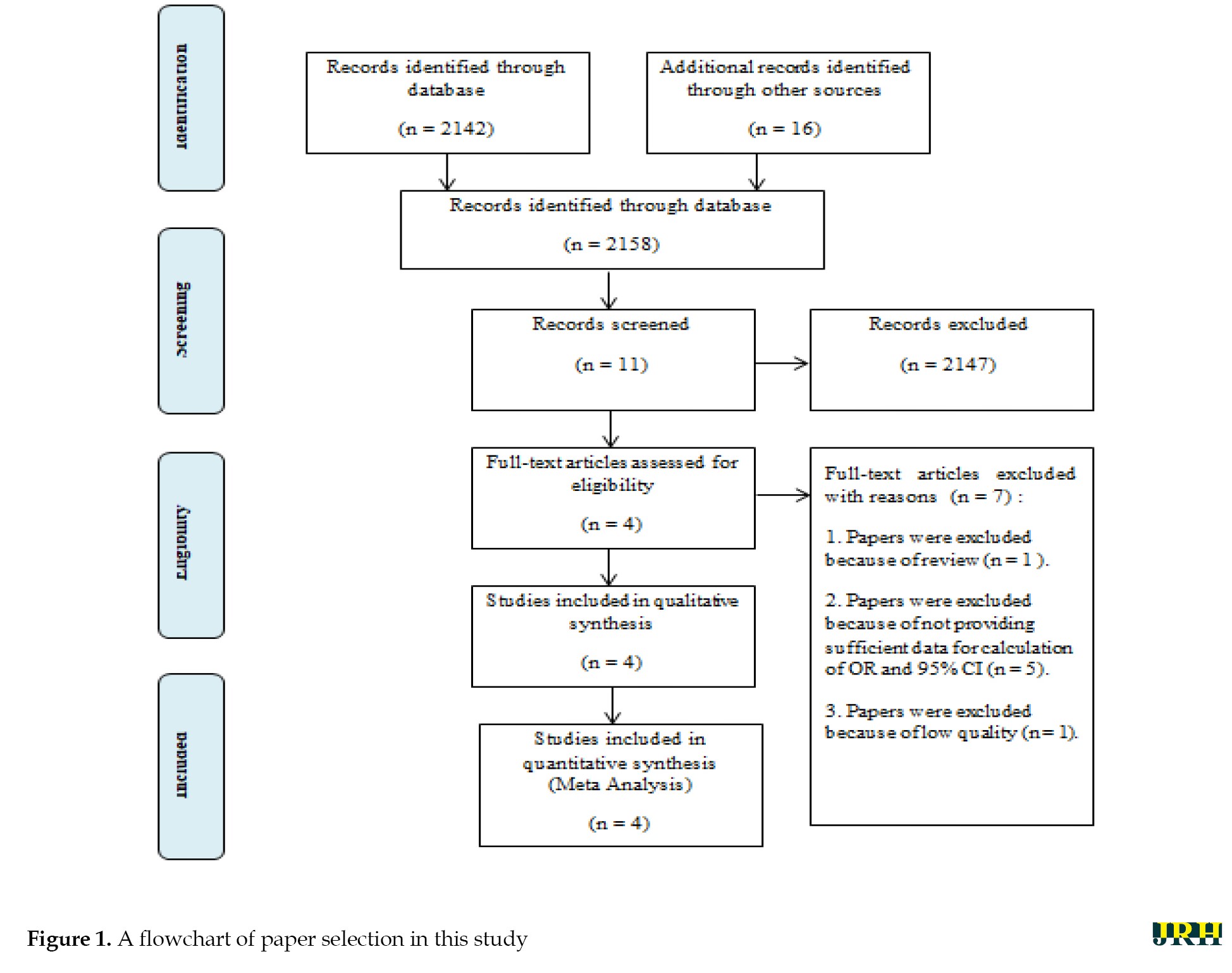
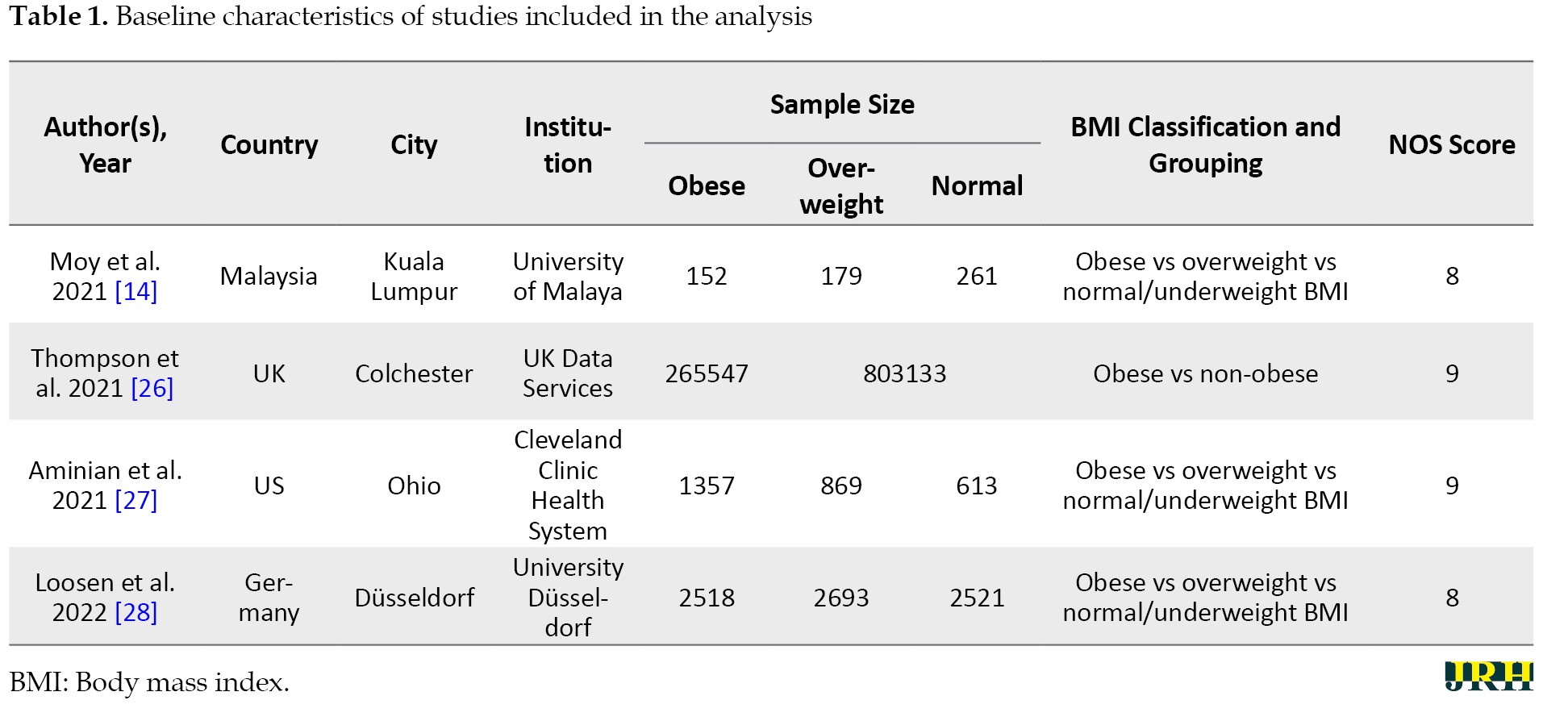
A total of 2158 potential studies were found, and 2147 studies were excluded due to inappropriate titles and abstracts. A further review of the full text of 11 potential studies was also carried out. Additionally, seven articles were excluded due to being reviews (n=1), inadequate data for calculating odds ratios and 95% CIs (n=5), and poor study quality (n=1). Finally, four studies were included in the meta-analysis. The study selection pathway is presented in Figure 1, while the characteristics of the papers are outlined and summarized in Tables 1 and 2, which detail the association between obesity, overweight, and long COVID in COVID-19 survivors.
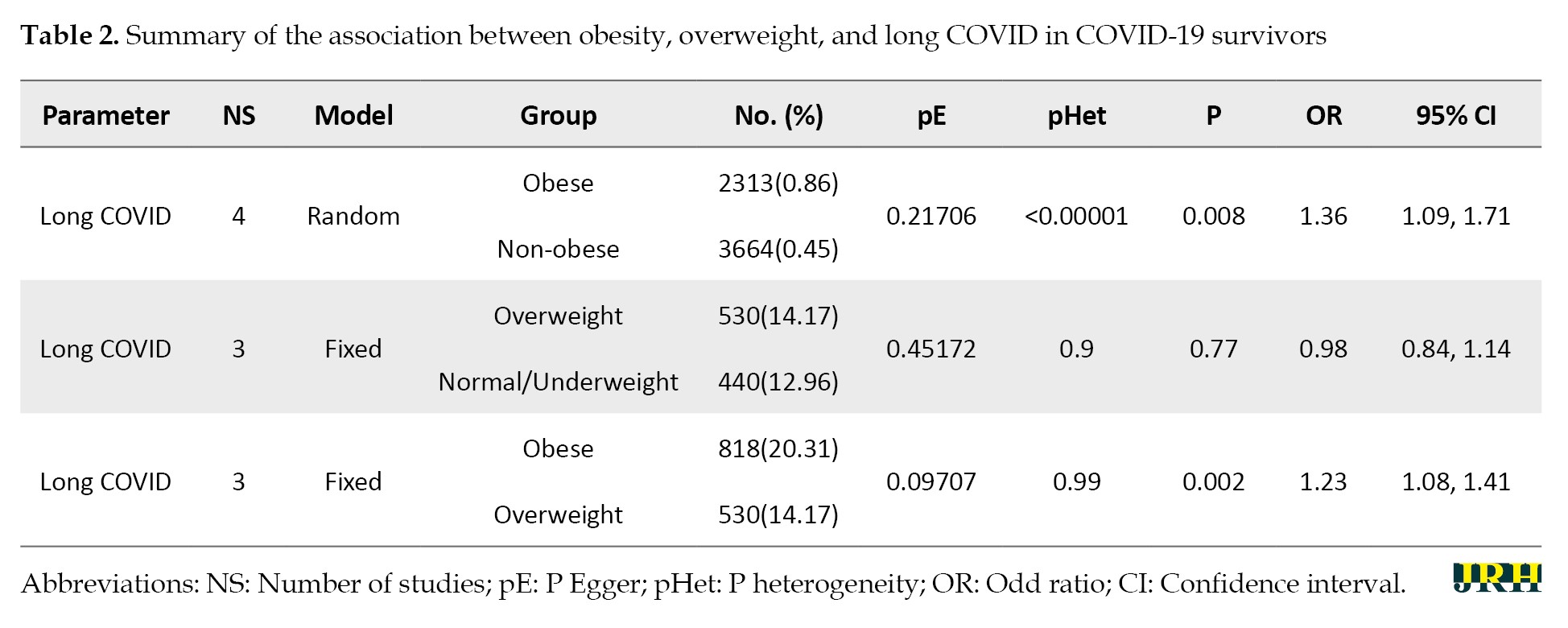
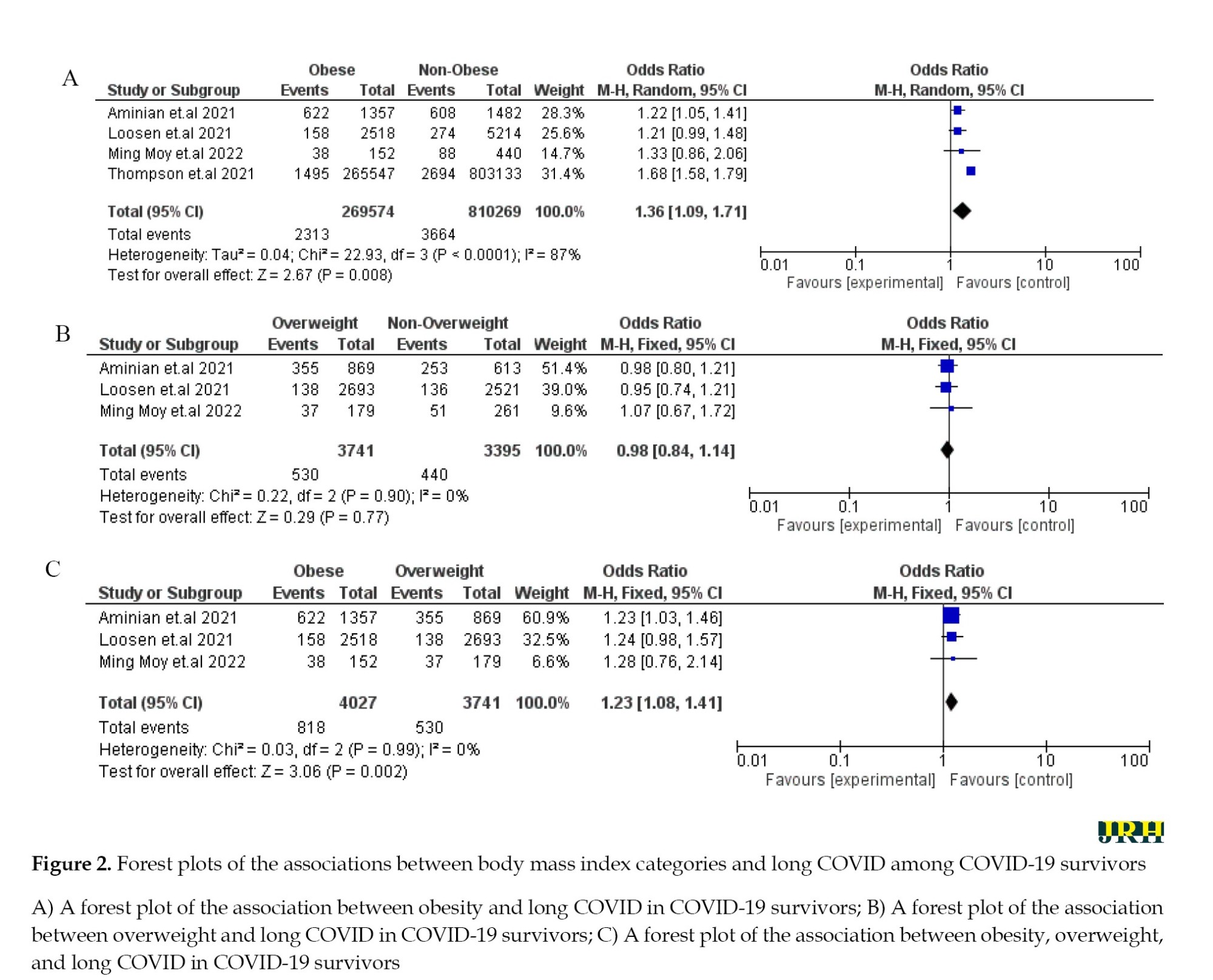
The long COVID incidence associated with obese and overweight populations in COVID-19 survivors
From four papers, two populations—namely, obese and overweight—were available for the meta-analysis. The evidence for long-term COVID incidence appeared significantly higher in COVID-19 survivors with obesity than those with no obesity (OR: 1.36; 95% CI: 1.09%, 1.71%). The result also showed that the risk of long-term COVID incidence increased in COVID-19 survivors with obesity than in those who were overweight (OR: 1.23; 95% CI, 1.08%, 1.41%). Moreover, COVID-19 survivors classified as overweight did not have a statistically significant difference in the incidence of long COVID compared to those with normal or underweight BMI (OR: 0.98; 95% CI: 0.84–1.14), suggesting that being overweight alone may not be a strong independent risk factor for developing long COVID (Figure 2).
Source of heterogeneity
In the analysis, heterogeneity was detected in all data, including the obese and overweight populations among COVID-19 survivors. Therefore, the fixed and random effect models were used to analyze the data. The fixed effect model was used for the analysis of overweight vs not overweight and obese vs overweight populations, as there was no evidence of heterogeneity. In contrast, the data on obese vs not obese populations were analyzed using the random effect model due to the presence of heterogeneity.
Potential publication bias
The potential for publication bias was analyzed using an Egger test [33]. The potential for publication bias (P<0.05) was not found concerning obese vs not obese, overweight vs not overweight, and obese vs overweight populations.
Discussion
The symptoms of long COVID are diverse and involve multiple organ systems, with the potential to impact virtually any part of the body. These manifestations can vary significantly among individuals and may fluctuate or develop differently over time. Long COVID can affect individuals across all age groups, genders, and ethnicities, including those who were previously healthy, fully vaccinated, or who experienced mild or asymptomatic acute infections. Nevertheless, individuals who experienced more severe acute illness, those with elevated BMI (overweight or obesity), and unvaccinated individuals are at an increased risk of developing long-term COVID [5]. Obesity has been linked to both an increased risk and greater severity of respiratory infections, as evidenced during the 2009 H1N1 swine flu pandemic and the more recent COVID-19 pandemic [19].
The findings of the meta-analysis in this study revealed a significant association between obesity and the development of long COVID in survivors of SARS-CoV-2 infection. In contrast, no significant relationship was found for overweight individuals. These results are consistent with multiple recent studies investigating post-COVID sequelae and the role of body weight and metabolic health. For example, a study in 2024 highlighted that 11% of participants transitioned to overweight or obese categories, and 42.5% of those who were already overweight or obese gained further weight during the early pandemic period [29]. These changes were linked to behaviors, such as solitary drinking, increased online food delivery, and prolonged online leisure, which were significantly associated with weight gain and stress—factors that could predispose individuals to long COVID through chronic inflammation and poor immune regulation [29]. Another study conducted in 2025 supports these insights by identifying sedentary behavior, unhealthy eating patterns, depression, and stress as key contributors to obesity during the pandemic [34]. These behavioral and emotional risk factors not only increase obesity but may also contribute to immune dysregulation, a proposed mechanism behind long COVID. Additionally, a 2024 study from Türkiye revealed a significant increase in overweight and obesity among NEET (not in education, employment, or training) youth post-pandemic [35].
This study showed that young females, particularly those disconnected from education and work, were more susceptible to unhealthy weight gain and potentially long-term health effects due to social isolation and economic instability—both conditions known to impact mental and physical health outcomes [34, 35]. These studies reinforce the current finding that obesity is not only a consequence of the COVID-19 pandemic but also a compounding factor in the development of long COVID. The absence of a significant association between overweight and long COVID in this present study may be due to less severe systemic inflammation in overweight individuals compared to those classified as obese. However, the trajectory of weight gain and worsening metabolic health across populations suggests that even overweight individuals may become at-risk over time, particularly if lifestyle changes persist post-pandemic.
Based on Vimercati et al., long COVID syndrome, whether mediated by inflammatory or non-inflammatory mechanisms, may result in significant functional impairments, limiting individuals’ ability to carry out daily activities at home and in the workplace [36]. Meanwhile, as the pathophysiology of long-term COVID-19 syndrome (LCS) is currently unclear, this finding provides important information about the possible pathophysiological relationship between metabolic risks and the development and severity of LCS. This supports the hypothesis that obesity-related chronic inflammation and immune-metabolic processes promote not only severe clinical courses of acute SARS-CoV-2 infection but also the development of LCS [37]. The risk of obesity-related comorbidities, such as type 2 diabetes, may be influenced by the duration of obesity, potentially due to prolonged exposure to chronic inflammation and metabolic dysregulation. These factors may also contribute to an increased susceptibility to SARS-CoV-2 infection among individuals with long-term obesity [19].
Obesity occurs because of a positive energy balance generally influenced by environmental, genetic, and behavioral factors [38]. Environmental factors, such as work duration, sleep duration, and other unsupportive environments for physical activities, are obesogenic [39, 40]. Concerning the immune response, there is a clear association between obesity and basal inflammatory status, characterized by higher circulating interleukin 6 and C-reactive protein levels [41]. Adipose tissue in obesity is “pro-inflammatory,” with increased expression of cytokines, particularly adipokines. There is also dysregulated expression of tissue leukocytes, and inflammatory macrophage (and innate lymphoid) subsets replace tissue regulatory (M2) phenotypic cells. In terms of host defense, obesity impairs adaptive immune responses to the influenza virus and conceivably could do so in COVID-19 [41-43].
Vimercati et al. revealed that other variables, such as systolic blood pressure (SBP), diastolic blood pressure (DBP), and cholesterol and triglyceride levels, exhibit no significant association with the onset of long COVID [36]. This indicates that traditional cardiac risk factors and cardiovascular pathophysiology may not adequately explain the etiopathogenesis of these persistent symptoms [36]. Consequently, individuals affected by overweight and obesity should be subjected to targeted active health surveillance. A BMI of more than 25 kg/m2 could be used as a threshold for attention in the clinical setting. The results of this exploratory study may lead to further research to identify the actual level of risk of LCS in a population stratified by BMI classes.
To the best of our knowledge, our study was the first to investigate the association between risk factors of obesity, overweight, normal weight/underweight, and long COVID symptoms in COVID-19 survivors. Since obesity and lipid disorders represent modifiable risk factors, our data suggest that lifestyle and metabolic interventions could be part of future strategies for pandemic preparedness.
Conclusion
Obesity has a significant association with COVID-19 survivors having a higher risk of developing long-term COVID conditions; overweight patients do not show a significant difference compared to those with normal weight or underweight. Our study also suggests that BMI can serve as an early diagnostic tool to identify individuals at the highest risk for developing long-term COVID-19 symptoms and as a predictor for COVID-19 prognosis.
Limitation
This study had several limitations that may have introduced bias. First, the limited number of included studies and the restriction to English-language publications may have led to selection and language bias. Second, although the Egger test did not reveal publication bias, the small sample size reduced the test’s sensitivity, and studies with null findings may have been underrepresented. Third, confounding bias is likely, as the meta-analysis lacked adjustment for key variables, such as age, gender, comorbidities, vaccination status, and disease severity, due to the absence of individual patient data. Additionally, variations in how long COVID and BMI were defined and measured across studies may have caused misclassification bias. Finally, despite the use of fixed and random effects models, significant heterogeneity in the included studies may have influenced the pooled effect estimates and the generalizability of the findings.
The meta-analysis controlled conflicting variables by applying strict inclusion criteria, selecting only moderate to high-quality studies based on the NQS, and using appropriate statistical models (fixed or random effects) depending on heterogeneity. While these methods helped reduce bias, individual-level confounders, like age, sex, comorbidities, and vaccination status, were not statistically adjusted due to data limitations— an issue acknowledged by the authors. Thus, further studies with a larger sample size are required to investigate the association.
Recommendation
Future research that combines obesity/overweight with other risk factors may provide a considerably better understanding of the association between obesity and patients with long COVID symptoms.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, methodology, and writing the original draft: Nuansa Firgie Paramitha; Data curation: Putu Ijiya Danta Awatara; Visualization: Yani Jane Rosihaningsih Soegiri; Investigation, validation, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus, first identified in Wuhan, China, in late 2019. This disease presents various symptoms, including fever, cough, fatigue, shortness of breath, anosmia, and dysgeusia. In severe cases, it can progress to pneumonia, kidney and liver dysfunction, and even death [1]. The morbidity burden of the COVID-19 pandemic is becoming increasingly apparent and concerning. Long COVID, also referred to as post-COVID-19 condition, is broadly characterized by the persistence of symptoms for three months or longer following the acute phase of COVID-19 infection [2]. Furthermore, long COVID has been defined as the persistence of symptoms, or the development of new symptoms, relating to SARS-CoV-2 infection late during COVID-19, occurring at least 28 days after diagnosis [3, 4].
In the early stages of the COVID-19 pandemic, many individuals experiencing long COVID faced challenges, such as being undiagnosed, dismissed, inadequately evaluated, or improperly treated. This phenomenon was often described by affected individuals as “medical gaslighting.” The term “long COVID” was first introduced by patients on May 20, 2020, to represent the prolonged sequelae and chronic complications associated with COVID-19 infection [5]. The prevalence of long-term COVID-19 is still uncertain, but evidence is emerging that it is relatively common [6]. Studies around the world have reported various incidence rates for long COVID with different follow-up examination times after the acute infection, including 76% of people at 6 months [7], 32.6% at 60 days [8], 87% at 60 days [9], and 96% at 90 days [10]. Data from the UK’s Office for National Statistics (ONS), based on a nationally representative non-institutionalized sample of lab-confirmed COVID-19 cases, including asymptomatic ones, estimated a prevalence of 11.7% at 12 weeks post-testing positive, increasing to 17.7% when considering only those symptomatic during the acute phase of the illness [6, 11].
Long COVID has been associated with a broad range of symptoms and health impacts [12-15]. Several systematic reviews have shown the most prevalent symptoms to be fatigue, shortness of breath, muscle pain, joint pain, headache, cough, chest pain, altered smell, altered taste, and diarrhea [15-18]. Long COVID predominantly affected individuals aged 35-65 years, with males representing the majority of cases [5]. Individuals with certain medical conditions, such as type 2 diabetes, allergies, a history of post-viral fatigue, asthma, chronic lung disease, heart failure, and chronic kidney disease, as well as those experiencing a more severe acute COVID-19 illness, with a high body mass index (BMI), or being unvaccinated, are at greater risk of developing long COVID. These groups are also more likely to experience severe and persistent symptoms [10]. Another meta-analysis identified overweight and obesity as potential risk factors for SARS-CoV-2 infection. However, this association was not consistently supported by serological data [19].
In the Lancet respiratory medicine, the PHOSP-COVID Collaborative Group reported the latest results from the UK-based, multicentre, prospective post-hospitalization COVID-19 (PHOSP-COVID) study, in which the investigators identified systemic inflammation and obesity as factors that might be associated with long COVID, representing potentially treatable traits in people with more severe post-COVID-19 symptoms [20]. The mechanisms underlying the long-term persistence of symptoms are unknown. A potential hypothesis is that the hyperinflammation associated with acute COVID-19 leads to a persistent inflammatory state following COVID-19, associated with dysregulated immunity and multiorgan dysfunction. Obesity also leads to local and systemic chronic inflammation, which in turn results in various chronic immune and metabolic diseases, such as type 2 diabetes, cancer, atherosclerosis, and inflammatory bowel disease. It can be hypothesized that obesity and obesity-related comorbidities may adversely affect the recovery of discharged patients with COVID-19 [20, 21]. Due to the evolving clinical spectrum of COVID-19, which is influenced by differences in variants, vaccines, and susceptibility by ethnicity and race, it is extremely challenging to define post-COVID-19 syndromes [22].
There are indications that metabolic dysfunction may promote or enhance these syndromes [22]. Identifying and understanding modifiable determinants associated with manifestations of long COVID, including obesity/overweight, would help adapt treatment pathways for particular phenotypes. Overweight and obesity are defined as abnormal or excessive fat accumulation that presents a health risk. A BMI of over 25-29.9 is considered overweight, while a BMI over 30 is classified as obese [23]. Nonetheless, collecting the best evidence regarding the temporal aspects of this post-viral syndrome and designing the most appropriate treatment approaches can be achieved. The direct implications of COVID-19 on health and well-being are well-discussed elsewhere; what remains to be seen is whether this pandemic is exacerbating the growing obesity pandemic. A systematic review and meta-analysis by Bakaloudi et al. suggest an overall global trend of weight gain during the first COVID-19 lockdown [24]. To date, no studies have assessed the indirect impact of the COVID-19 pandemic on obesity and overweight risk factors that could explain this trend. Therefore, this paper managed to fill this gap by describing the effects of the COVID-19 pandemic and the needed countermeasures on obesity risk factors to explore the underlying mechanisms of the general trend of weight gain during the COVID-19 pandemic. In this study, the association between obesity, overweight, and long COVID in COVID-19 survivors was investigated.
Methods
Research design
This meta-analysis, conducted from March 2022 to August 2022, investigated the association between obese and overweight populations and the incidence of long-term COVID-19 in COVID-19 survivors. Preferred reporting items for systematic review and meta-analysis (PRISMA) were applied as the framework of this study [25].
Eligibility criteria
All data were obtained from articles published in PubMed, PMC, ScienceDirect, and Taylor and Francis. Potential outcomes were thoroughly identified, and a further search was conducted to obtain the papers that might be included in this study. The keywords were as follows: [“Obesity” or “overweight”] and [“long Covid” or “post-acute COVID-19 syndrome”] [26–28]. Two independent researchers (Nuansa Firgie Paramitha and Putu Ijiya Danta Awatara) extracted data using a pilot form, and further discussion was held if there was any disagreement. The articles included in this study were those that met the following inclusion criteria:
Population: Studies involving human participants aged 19 years or older, as specified in the study by Lee & Yoo [29].
Exposure: Studies must report pre-pandemic and post-pandemic BMI values or clearly define overweight (BMI 23–24.9 kg/m²) and obesity (BMI ≥25 kg/m²), consistent with the guidelines of the Asia-Pacific World Health Organization (WHO) or the Korean Society for the Study of Obesity (KSSO) referenced in the study.
Outcome: Studies assessing long COVID outcomes, defined as symptoms persisting for ≥12 weeks after acute COVID-19 infection, in line with WHO definitions.
Study type: Observational studies (cross-sectional, case-control, and cohort), either prospective or retrospective, that include effect size estimates (e.g. odd ratio, risk ratio) or data that allow calculation of the association between BMI status and long COVID development.
Publication date: Articles published between January 2020 and December 2024, to reflect the COVID-19 pandemic period covered in the referenced study.
Language: Articles must be published in English.
Availability: Full-text access must be available for data extraction.
Methodological quality: Studies must have moderate to high methodological quality, as assessed by the Newcastle-Ottawa scale (NOS), with a score of 5 or higher. This assessment focused on the rigor of the study design, including participant selection, comparability of groups, and outcome/exposure ascertainment.
Papers were excluded based on the following criteria:
Pediatric populations: Studies that exclusively involved participants under 19 years of age.
No long COVID data: Studies that did not measure or define long COVID or persistent symptoms post-infection.
No BMI classification: Studies that did not include clear definitions or measures of overweight or obesity.
Interventional trials or pharmacological studies: These were excluded unless baseline BMI and long COVID data were explicitly reported and analyzable.
Case reports, reviews, editorials, and conference abstracts: Non-original data or studies without full datasets were excluded.
Non-human or animal studies: Only studies involving human subjects were included.
High risk of bias: Studies assessed to have a high risk of bias using standardized tools (NOS) were excluded from quantitative synthesis.
Duplicate publications: In cases of multiple reports from the same dataset, only the most comprehensive or recent version was included.
Assessment of the article quality
Before the analysis process, the quality of the papers was assessed using the NOS. In this assessment, the evaluation of patient selection, group comparisons, and exposure assessment was performed. A score of less than 4 indicated that the paper had low quality, while a score of 5-6 indicated moderate quality. Papers with a score greater than or equal to 7 were considered high quality [30]. Articles included in this analysis were those of moderate to high quality. Two independent investigators (Nuansa Firgie Paramitha and Putu Ijiya Danta Awatara) conducted the study assessment using a pilot form. Other investigators (Putu Ijiya Danta Awatara) provided consultation if a disagreement arose.
Study measures
The predictor variable in this study was the obese and overweight populations among COVID-19 survivors. COVID-19 survivors are individuals who tested positive for the coronavirus and/or were later confirmed to have had the virus by testing positive for antibodies [31]. Overweight and obesity are defined as abnormal or excessive fat accumulation that may impair health. BMI is a simple weight-for-height index commonly used to classify overweight and obesity in adults. It is defined as a person’s weight in kilograms divided by the square of his/her height in meters (kg/m2). For adults, the WHO defines overweight and obesity as follows: Underweight is a BMI less than 18.5, norm weight is a BMI between 18.5 and 24.9, overweight is a BMI greater than or equal to 25-29.9, and obesity is a BMI greater than or equal to 30 [32].
Statistical analysis
The comparison of obese and overweight populations in COVID-19 survivors was performed using a Z test, and their effects on the incidence of long COVID were determined by the calculation of the odds ratio and 95% confidence interval (CI). The data with potential bias and heterogeneity across the studies were assessed first before evaluating the correlation and effect estimates. Publication bias was analyzed using an Egger test, with the potential for publication bias considered if the P<0.05. Furthermore, heterogeneity among studies was assessed using a Q test, with data considered heterogeneous if the P<0.10. The calculation for our meta-analysis used Comprehensive Meta-Analysis software, version 3 (CMA, Chicago, US).
Results
Eligible studies
A total of 2158 potential studies were found, and 2147 studies were excluded due to inappropriate titles and abstracts. A further review of the full text of 11 potential studies was also carried out. Additionally, seven articles were excluded due to being reviews (n=1), inadequate data for calculating odds ratios and 95% CIs (n=5), and poor study quality (n=1). Finally, four studies were included in the meta-analysis. The study selection pathway is presented in Figure 1, while the characteristics of the papers are outlined and summarized in Tables 1 and 2, which detail the association between obesity, overweight, and long COVID in COVID-19 survivors.


The long COVID incidence associated with obese and overweight populations in COVID-19 survivors
From four papers, two populations—namely, obese and overweight—were available for the meta-analysis. The evidence for long-term COVID incidence appeared significantly higher in COVID-19 survivors with obesity than those with no obesity (OR: 1.36; 95% CI: 1.09%, 1.71%). The result also showed that the risk of long-term COVID incidence increased in COVID-19 survivors with obesity than in those who were overweight (OR: 1.23; 95% CI, 1.08%, 1.41%). Moreover, COVID-19 survivors classified as overweight did not have a statistically significant difference in the incidence of long COVID compared to those with normal or underweight BMI (OR: 0.98; 95% CI: 0.84–1.14), suggesting that being overweight alone may not be a strong independent risk factor for developing long COVID (Figure 2).
Source of heterogeneity
In the analysis, heterogeneity was detected in all data, including the obese and overweight populations among COVID-19 survivors. Therefore, the fixed and random effect models were used to analyze the data. The fixed effect model was used for the analysis of overweight vs not overweight and obese vs overweight populations, as there was no evidence of heterogeneity. In contrast, the data on obese vs not obese populations were analyzed using the random effect model due to the presence of heterogeneity.
Potential publication bias
The potential for publication bias was analyzed using an Egger test [33]. The potential for publication bias (P<0.05) was not found concerning obese vs not obese, overweight vs not overweight, and obese vs overweight populations.
Discussion
The symptoms of long COVID are diverse and involve multiple organ systems, with the potential to impact virtually any part of the body. These manifestations can vary significantly among individuals and may fluctuate or develop differently over time. Long COVID can affect individuals across all age groups, genders, and ethnicities, including those who were previously healthy, fully vaccinated, or who experienced mild or asymptomatic acute infections. Nevertheless, individuals who experienced more severe acute illness, those with elevated BMI (overweight or obesity), and unvaccinated individuals are at an increased risk of developing long-term COVID [5]. Obesity has been linked to both an increased risk and greater severity of respiratory infections, as evidenced during the 2009 H1N1 swine flu pandemic and the more recent COVID-19 pandemic [19].
The findings of the meta-analysis in this study revealed a significant association between obesity and the development of long COVID in survivors of SARS-CoV-2 infection. In contrast, no significant relationship was found for overweight individuals. These results are consistent with multiple recent studies investigating post-COVID sequelae and the role of body weight and metabolic health. For example, a study in 2024 highlighted that 11% of participants transitioned to overweight or obese categories, and 42.5% of those who were already overweight or obese gained further weight during the early pandemic period [29]. These changes were linked to behaviors, such as solitary drinking, increased online food delivery, and prolonged online leisure, which were significantly associated with weight gain and stress—factors that could predispose individuals to long COVID through chronic inflammation and poor immune regulation [29]. Another study conducted in 2025 supports these insights by identifying sedentary behavior, unhealthy eating patterns, depression, and stress as key contributors to obesity during the pandemic [34]. These behavioral and emotional risk factors not only increase obesity but may also contribute to immune dysregulation, a proposed mechanism behind long COVID. Additionally, a 2024 study from Türkiye revealed a significant increase in overweight and obesity among NEET (not in education, employment, or training) youth post-pandemic [35].
This study showed that young females, particularly those disconnected from education and work, were more susceptible to unhealthy weight gain and potentially long-term health effects due to social isolation and economic instability—both conditions known to impact mental and physical health outcomes [34, 35]. These studies reinforce the current finding that obesity is not only a consequence of the COVID-19 pandemic but also a compounding factor in the development of long COVID. The absence of a significant association between overweight and long COVID in this present study may be due to less severe systemic inflammation in overweight individuals compared to those classified as obese. However, the trajectory of weight gain and worsening metabolic health across populations suggests that even overweight individuals may become at-risk over time, particularly if lifestyle changes persist post-pandemic.
Based on Vimercati et al., long COVID syndrome, whether mediated by inflammatory or non-inflammatory mechanisms, may result in significant functional impairments, limiting individuals’ ability to carry out daily activities at home and in the workplace [36]. Meanwhile, as the pathophysiology of long-term COVID-19 syndrome (LCS) is currently unclear, this finding provides important information about the possible pathophysiological relationship between metabolic risks and the development and severity of LCS. This supports the hypothesis that obesity-related chronic inflammation and immune-metabolic processes promote not only severe clinical courses of acute SARS-CoV-2 infection but also the development of LCS [37]. The risk of obesity-related comorbidities, such as type 2 diabetes, may be influenced by the duration of obesity, potentially due to prolonged exposure to chronic inflammation and metabolic dysregulation. These factors may also contribute to an increased susceptibility to SARS-CoV-2 infection among individuals with long-term obesity [19].
Obesity occurs because of a positive energy balance generally influenced by environmental, genetic, and behavioral factors [38]. Environmental factors, such as work duration, sleep duration, and other unsupportive environments for physical activities, are obesogenic [39, 40]. Concerning the immune response, there is a clear association between obesity and basal inflammatory status, characterized by higher circulating interleukin 6 and C-reactive protein levels [41]. Adipose tissue in obesity is “pro-inflammatory,” with increased expression of cytokines, particularly adipokines. There is also dysregulated expression of tissue leukocytes, and inflammatory macrophage (and innate lymphoid) subsets replace tissue regulatory (M2) phenotypic cells. In terms of host defense, obesity impairs adaptive immune responses to the influenza virus and conceivably could do so in COVID-19 [41-43].
Vimercati et al. revealed that other variables, such as systolic blood pressure (SBP), diastolic blood pressure (DBP), and cholesterol and triglyceride levels, exhibit no significant association with the onset of long COVID [36]. This indicates that traditional cardiac risk factors and cardiovascular pathophysiology may not adequately explain the etiopathogenesis of these persistent symptoms [36]. Consequently, individuals affected by overweight and obesity should be subjected to targeted active health surveillance. A BMI of more than 25 kg/m2 could be used as a threshold for attention in the clinical setting. The results of this exploratory study may lead to further research to identify the actual level of risk of LCS in a population stratified by BMI classes.
To the best of our knowledge, our study was the first to investigate the association between risk factors of obesity, overweight, normal weight/underweight, and long COVID symptoms in COVID-19 survivors. Since obesity and lipid disorders represent modifiable risk factors, our data suggest that lifestyle and metabolic interventions could be part of future strategies for pandemic preparedness.
Conclusion
Obesity has a significant association with COVID-19 survivors having a higher risk of developing long-term COVID conditions; overweight patients do not show a significant difference compared to those with normal weight or underweight. Our study also suggests that BMI can serve as an early diagnostic tool to identify individuals at the highest risk for developing long-term COVID-19 symptoms and as a predictor for COVID-19 prognosis.
Limitation
This study had several limitations that may have introduced bias. First, the limited number of included studies and the restriction to English-language publications may have led to selection and language bias. Second, although the Egger test did not reveal publication bias, the small sample size reduced the test’s sensitivity, and studies with null findings may have been underrepresented. Third, confounding bias is likely, as the meta-analysis lacked adjustment for key variables, such as age, gender, comorbidities, vaccination status, and disease severity, due to the absence of individual patient data. Additionally, variations in how long COVID and BMI were defined and measured across studies may have caused misclassification bias. Finally, despite the use of fixed and random effects models, significant heterogeneity in the included studies may have influenced the pooled effect estimates and the generalizability of the findings.
The meta-analysis controlled conflicting variables by applying strict inclusion criteria, selecting only moderate to high-quality studies based on the NQS, and using appropriate statistical models (fixed or random effects) depending on heterogeneity. While these methods helped reduce bias, individual-level confounders, like age, sex, comorbidities, and vaccination status, were not statistically adjusted due to data limitations— an issue acknowledged by the authors. Thus, further studies with a larger sample size are required to investigate the association.
Recommendation
Future research that combines obesity/overweight with other risk factors may provide a considerably better understanding of the association between obesity and patients with long COVID symptoms.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, methodology, and writing the original draft: Nuansa Firgie Paramitha; Data curation: Putu Ijiya Danta Awatara; Visualization: Yani Jane Rosihaningsih Soegiri; Investigation, validation, review and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Nurkasanah S, Dewi CC, Qosiani EJ, Kholifatin DA, Anggita ES. Analysis of voluntary blood donors’ characteristics during the third-peak of COVID-19 in Bojonegoro regency, Indonesia. Journal of Community Empowerment for Health (JCOEMPH). 2023; 6(1):13-17. [DOI:10.22146/jcoemph.80232]
- Greenhalgh T, Sivan M, Perlowski A, Nikolich JŽ. Long COVID: A clinical update. The Lancet. 2024; 404(10453):707-24. [DOI:10.1016/S0140-6736(24)01136-X] [PMID]
- Nabavi N. Long covid: How to define it and how to manage it. BMJ. 2020; 370:m3489. [DOI:10.1136/bmj.m3489] [PMID]
- Mendelson M, Nel J, Blumberg L, Madhi SA, Dryden M, Stevens W, et al. Long-COVID: An evolving problem with an extensive impact. South African Medical Journal. 2020; 111(1):10-12. [DOI:10.7196/SAMJ.2020.v111i11.15433] [PMID]
- Callard F, Perego E. How and why patients made long Covid. Social Science & Medicine. 2021; 268:113426. [DOI:10.1016/j.socscimed.2020.113426] [PMID]
- Ziauddeen N, Gurdasani D, O’Hara ME, Hastie C, Roderick P, Yao G, et al. Characteristics and impact of long covid: Findings from an online survey. PLoS One. 2022; 17(3):e0264331. [DOI:10.1371/journal.pone.0264331] [PMID]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. The Lancet. 2021; 397(10270):220-32. [DOI:10.1016/S0140-6736(20)32656-8] [PMID]
- Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Annals of Internal Medicine. 2021; 174(4):576-8. [DOI:10.7326/M20-5661] [PMID]
- Carfì A, Bernabei R, Landi F; Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020; 324(6):603-5. [DOI:10.1001/jama.2020.12603] [PMID]
- Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021; 38:101019. [DOI:10.1016/j.eclinm.2021.101019] [PMID]
- Office for National Statistics. Technical article: Updated estimates of the prevalence of post-acute symptoms among people with Coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. Newport: Office for National Statistics; 2021. [Link]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021; 27(4):601-15. [DOI:10.1038/s41591-021-01283-z] [PMID]
- Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA. 2020; 324(17):1723-4.[DOI:10.1001/jama.2020.19719] [PMID]
- Moy FM, Hairi NN, Lim ERJ, Bulgiba A. Long COVID and its associated factors among COVID survivors in the community from a middle-income country-An online cross-sectional study. PLoS One. 2022; 17(8):e0273364. [DOI:10.1371/journal.pone.0273364] [PMID]
- Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of post acute sequelae of SARS-CoV-2 Infection: A systematic review. JAMA Network Open. 2021; 4(10):e2128568. [DOI:10.1001/jamanetworkopen.2021.28568] [PMID]
- Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long COVID: A review. Journal of The Royal Society of Medicine. 2021; 114(9):428-42. [DOI:10.1177/01410768211032850] [PMID]
- Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Scientific Reports. 2021; 11(1):16144. [DOI:10.1038/s41598-021-95565-8] [PMID]
- Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: A living systematic review. BMJ Global Health. 2021; 6(9):e005427. [DOI:10.1136/bmjgh-2021-005427] [PMID]
- Loef B, Boer JMA, Beekman M, Campman SL, Hoogendijk EO, Huider F, Pagen DME, et al. The association of overweight, obesity, and long-term obesity with SARS-CoV-2 infection: A meta-analysis of 9 population-based cohorts from the Netherlands cohorts consortium. International Journal of Obesity. 2025; 49(4):586-95. [DOI:10.1038/s41366-024-01660-x] [PMID]
- PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. The Lancet. Respiratory Medicine. 2022; 10(8):761-75. [DOI:10.1016/S2213-2600(22)00127-8] [PMID]
- Shang L, Wang L, Zhou F, Li J, Liu Y, Yang S. Long‐term effects of obesity on COVID‐19 patients discharged from hospital. Immunity, Inflammation and Disease. 2021; 9(4):1678-85. [DOI:10.1002/iid3.522] [PMID]
- Scherer PE, Kirwan JP, Rosen CJ. Post-acute sequelae of COVID-19: A metabolic perspective. Elife. 2022; 11:e78200.[DOI:10.7554/eLife.78200] [PMID]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363(9403):157-63. [DOI:10.1016/S0140-6736(03)15268-3] [PMID]
- Bakaloudi DR, Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Chourdakis M. Impact of the first COVID-19 lockdown on body weight: A combined systematic review and a meta-analysis. Clinical Nutrition. 2022; 41(12):3046-54. [PMID]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Moher D, Liberati A, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Plos Medicine. 2009; 6(7):e1000097. [DOI:10.1371/journal.pmed.1000097] [PMID]
- Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nature Communications. 2022; 13(1):3528. [DOI:10.1038/s41467-022-30836-0] [PMID]
- Aminian A, Bena J, Pantalone KM, Burguera B. Association of obesity with postacute sequelae of COVID‐19. Diabetes, Obesity & Metabolism. 2021; 23(9):2183-8. [DOI:10.1111/dom.14454] [PMID]
- Loosen SH, Jensen BO, Tanislav C, Luedde T, Roderburg C, Kostev K. Obesity and lipid metabolism disorders determine the risk for development of long COVID syndrome: A cross-sectional study from 50,402 COVID-19 patients. Infection. 2022; 50(5):1165-70. [DOI:10.1007/s15010-022-01784-0] [PMID]
- Lee J, Yoo S. Weight gain, new-onset overweight or obesity, and their influencing factors during the social distancing era of the COVID-19 pandemic. Heliyon. 2024; 10(15):e34733.[DOI:10.1016/j.heliyon.2024.e34733] [PMID]
- Luchini C, Veronese N, Nottegar A, Shin J Il, Gentile G, Granziol U, et al. Assessing the quality of studies in meta‐research: Review/guidelines on the most important quality assessment tools. Pharmaceutical Statistics. 2021; 20(1):185-95. [DOI:10.1002/pst.2068] [PMID]
- Prioleau T. Learning from the experiences of COVID-19 survivors: Web-based survey study. JMIR Formative Research. 2021; 5(5):e23009. [DOI:10.2196/23009] [PMID]
- Fruh SM. Obesity: Risk factors, complications, and strategies for sustainable long‐term weight management. Journal of the American Association of Nurse Practitioners. 2017; 29(S1):S3-14. [DOI:10.1002/2327-6924.12510] [PMID]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109):629-34. [DOI:10.1136/bmj.315.7109.629] [PMID]
- Nour TY, Altintaş KH. Effect of the COVID-19 pandemic on obesity and its risk factors: A systematic review. BMC Public Health. 2023; 23(1):1018. [DOI:10.1186/s12889-023-15833-2] [PMID]
- Karaoglan D, Begen N, Tat P. The impact of the COVID-19 pandemic on overweight and obesity: The case of NEET in Türkiye. Discover Public Health. 2024; 21:182. [DOI:10.1186/s12982-024-00297-5]
- Vimercati L, De Maria L, Quarato M, Caputi A, Gesualdo L, Migliore G, et al. Association between Long COVID and Overweight/Obesity. Journal of Clinical Medicine. 2021; 10(18):4143. [DOI:10.3390/jcm10184143] [PMID]
- Chiappetta S, Sharma AM, Bottino V, Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. International Journal of Obesity. 2020; 44(8):1790-2.[DOI:10.1038/s41366-020-0597-4] [PMID]
- Omer T. The causes of obesity: An in-depth review. Advances in Obesity, Weight Management & Control. 2020; 10(3):90-4. [DOI:10.15406/aowmc.2020.10.00312]
- Robinson E, Boyland E, Chisholm A, Harrold J, Maloney NG, Marty L, et al. Obesity, eating behavior and physical activity during COVID-19 lockdown: A study of UK adults. Appetite. 2021; 156:104853. [DOI:10.1016/j.appet.2020.104853] [PMID]
- Dunton GF, Do B, Wang SD. Early effects of the COVID-19 pandemic on physical activity and sedentary behavior in children living in the U.S. BMC Public Health. 2020; 20(1):1351.[DOI:10.1186/s12889-020-09429-3] [PMID]
- Sattar N, McInnes IB, McMurray JJV. Obesity Is a risk factor for severe covid-19 infection: Multiple potential mechanisms. Circulation. 2020; 142(1):4-6. [DOI:10.1161/CIRCULATIONAHA.120.047659] [PMID]
- El-Saber Batiha G, Magdy Beshbishy A, Stephen Adeyemi O, Nadwa E, Rashwan E, Yokoyama N, et al. Safety and efficacy of hydroxyurea and eflornithine against most blood parasites Babesia and Theileria. PLoS One. 2020; 15(2):e0228996. [DOI:10.1371/journal.pone.0228996] [PMID]
- Magdy Beshbishy A, Hetta HF, Hussein DE, Saati AA, C Uba C, Rivero-Perez N, et al. Factors associated with increased morbidity and mortality of obese and overweight covid-19 patients. Biology (Basel). 2020; 9(9):280. [DOI:10.3390/biology9090280] [PMID]
Type of Study: Orginal Article |
Subject:
● Health Education
Received: 2024/09/7 | Accepted: 2025/05/19 | Published: 2025/11/1
Received: 2024/09/7 | Accepted: 2025/05/19 | Published: 2025/11/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |