Volume 15, Issue 6 (Nov & Dec 2025)
J Research Health 2025, 15(6): 559-570 |
Back to browse issues page
Ethics code: IR.GMU.REC.1400.215
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dogonchi M, Moshki M, Saberi Noghabi E, Mohammadzadeh F. The Role of Health Locus of Control and Perceived Social Support in Performing Breast Cancer Screening: A Cross-sectional Study in Iran. J Research Health 2025; 15 (6) :559-570
URL: http://jrh.gmu.ac.ir/article-1-2657-en.html
URL: http://jrh.gmu.ac.ir/article-1-2657-en.html
1- Department of Health Education and Health Promotion, Social Development and Health Promotion Research Center, Faculty of Health, Gonabad University of Medical Sciences, Gonabad, Iran.
2- Department of Community Health Nursing, Faculty of Nursing, Gonabad University of Medical Sciences, Gonabad, Iran.
3- Department of Biostatistics and Epidemiology, Social Development and Health Promotion Research Center, Faculty of Health, Gonabad University of Medical Sciences, Gonabad, Iran. ,fmhz.uni@gmail.com
2- Department of Community Health Nursing, Faculty of Nursing, Gonabad University of Medical Sciences, Gonabad, Iran.
3- Department of Biostatistics and Epidemiology, Social Development and Health Promotion Research Center, Faculty of Health, Gonabad University of Medical Sciences, Gonabad, Iran. ,
Full-Text [PDF 650 kb]
(242 Downloads)
| Abstract (HTML) (2240 Views)
Full-Text: (278 Views)
Introduction
Breast cancer is the most common malignancy known in the world [1]. The projections from global burden of breast cancer surveillance studies indicate that the number of new cases is expected to increase by 40%, reaching more than three million. In addition, breast cancer deaths will increase by 50% to one million by 2040 [2]. In Iran, breast cancer ranks first in terms of incidence and fifth in terms of mortality, accounting for 13% of all cancers regardless of gender, with an age-standardized incidence rate (ASIR) of 35.8 and an age-standardized mortality rate (ASMR) of 10.8 per 100,000 women, which is currently lower than neighboring countries with similar income levels. This rate has increased compared to previous reports; thus, in the latest published report of Iran’s national cancer registration program, the incidence rate was 34.5 per 100,000 women [3, 4]. Currently, the incidence of this cancer is increasing in Iran, and unfortunately, women in Iran are at advanced stages of breast cancer at a young age [3].
If this cancer is detected in the early stages using screening methods, it can be treated [5]. Screening methods for the early diagnosis of this deadly disease include mammography, monthly self-examination, and clinical examinations [6]. Mammography is recommended for women over 40 years old, while clinical breast examinations should be conducted every three years for women between 20 and 40 years old, and annually by a specialist for those over 40. Additionally, breast self-examination is advised for women over 20 years old on a monthly basis [7]. Studies have revealed that people have a low level of awareness regarding breast cancer screening coverage. When women are more aware of breast cancer, they are more likely to undergo screening, which can have a positive impact on their health. Awareness refers to the ability to recall details, understand general procedures, recognize processes, identify patterns, and comprehend structures or situations related to breast cancer screening [8].
Promoting preventive behaviors will improve people’s performance, increase their quality of life, and reduce healthcare costs [9]. Research has found that improving knowledge, social support, and a high health locus of control can encourage healthy behaviors [10-12]. One factor that promotes health-related behaviors in a person is the center of health control [13]. Health locus of control is the extent of a person’s control over certain events in his/her life, which ultimately predicts health behavior based on people’s beliefs [14]. These factors include the following:
The internal health locus of control (IHLC) encompasses the degree to which a person believes that their internal factors [15] and behaviors are responsible for their health and illness. The control axis of effective people includes the extent to which a person believes that other individuals determine his/her health. The axis of luck control includes the degree of a person’s belief that his/her health depends on his/her luck, fortune, and destiny [15]. People who rely more on themselves have poorer cooperation with healthcare providers. The concept examined through the health locus of control is the perception of personal effectiveness and individual responsibility regarding health [16]. The health locus of control has been shown to be positively related to engagement in health behaviors [17]. Understanding the mechanisms of this relationship could help identify groups that may benefit from targeting certain mediators. Social support could mediate the relationship between IHLC and health behaviors, such as physical activity, dietary practices, and screening [18]. The health locus of control is associated with better health behaviors through the mediating effect of social support. The persuasion and motivation of those around individuals with higher IHLC positively affected their ability to engage in certain health behaviors [19, 20]. In addition, the results of other studies show that social support also plays a key role in performing health behaviors and screening [16, 21-23]. Social support provides access to information, encourages people, and helps them adopts preventive behaviors. Social support through increasing self-efficacy leads to overcoming perceived obstacles (emotional, logical, and financial) in breast cancer screening [24, 25]. Social support refers to the awareness that a person is part of a society that loves and values him/her. Social support includes tangible components, such as financial and physical assistance, and intangible elements, such as encouragement and guidance. This support can have different forms. There are four main types of social support. Emotional support includes offering sympathy, love, trust, and care. Instrumental support means a tangible help that a person needs. Informational support means providing advice, comments, and information that a person can use when facing a problem. Evaluation support involves providing valuable information for self-assessment [26].
Given that health control beliefs and perceived social support play an important role in health behaviors [27], and considering the growing trend of breast cancer in Iran, along with the referral of many breast cancer patients in advanced stages of the disease, it is crucial to reflect on and address this issue to promote behaviors that lead to the early diagnosis of breast cancer in order to reduce mortality associated with it [28]. Therefore, the present study aimed to investigate the role of health control beliefs and perceived social support in breast cancer screening in women covered by comprehensive health service centers in Gonabad (Northeast Iran) in 2022.
Methods
Design, settings, and participants
This cross-sectional study was conducted on women referred by the comprehensive health service centers in Gonabad, located in northeastern Iran, from February 2022 to March 2023. The inclusion criteria were being 20 years or older and providing informed consent to participate, with no physical or mental problems, and no history of breast self-examination in the study. Women with current or suspected diagnoses of breast cancer or those with incomplete questionnaires were excluded from the study. Based on the sample size formula (Equation 1):
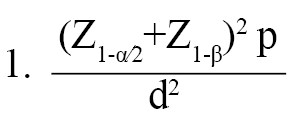
, a type 1 error (α) of 0.05, a test power (β) of 0.80, and an error (d) of 0.05, the sample size required to estimate the prevalence of breast cancer screening in Iranian women, based on previous studies [27-29] suggesting a 10% prevalence, was initially calculated to be 283 samples. To account for a potential 10% drop in participation, the sample size was increased to 311. This sample size was deemed adequate for logistic regression analysis based on the rule of events per variable (EPV). It is recommended that an EPV of 10 is acceptable for logistic regression [30].
The sampling method was a stratified random sample proportional to the population size. In this approach, each of the comprehensive health service centers in Gonabad City was considered a stratum, and samples were selected through simple random sampling from the list of women aged 20 and above in each center, proportional to the population served and the determined sample size.
Instruments
Data were collected using self-administered questionnaires.
Demographic checklist
It included several items on age, marital age, age at first delivery, marital status, education level, job, income level, number of children, breastfeeding duration, health insurance coverage, family history of breast disease, and some items about breast cancer screening behaviors and their barriers.
Breast cancer knowledge research-made questionnaire
This questionnaire included questions categorized under three main topics: Breast cancer potential risk factors, signs and symptoms, and cancer screening and prevention. To ensure the questionnaire’s validity, a committee of experts in research methodology, obstetrics and gynecology, and oncology reviewed and approved the questions. A pilot study involving 30 participants was conducted to test the questionnaire’s clarity and reliability. The reliability of the questionnaire, measured using the Kuder-Richardson coefficient, was 0.705. Respondents were asked to select their answers from ‘yes’, ‘no’, or ‘don’t know’. These responses were then dichotomized, with correct answers receiving a score of 1 and ‘don’t know’ or incorrect answers receiving a score of 0. Each participant’s total knowledge score was calculated by summing their responses, with a maximum possible score of 10.
Based on the quartiles, participants’ total knowledge scores were categorized as follows: inferior knowledge (score of 0–2.5), poor knowledge (score of 2.6–5), fair knowledge (score of 5.1–7.5), and good knowledge (score of 7.5–10).
Breast cancer screening behaviors and their barriers research-made questionnaire
This scale included six items concerning breast cancer screening methods (self-examination, clinical examinations, and mammography) and barriers to women’s breast cancer screening. For example, it asked, “Do you perform breast self-examination monthly? If no, what obstacles do you face when it comes to performing breast self-examinations?” The reliability of this questionnaire, measured using the Kuder-Richardson coefficient, was 0.705.
Multidimensional health locus of control scale (MHLCS)
The MHLCS was developed in 1987 by Wallston et al. [31] to assess individuals’ beliefs about their control over their health. This questionnaire measures three main domains: Powerful others health locus of control (PHLC), IHLC, and chance health locus of control (CHLC). PHLC refers to the belief that an individual’s health is affected by external factors. IHLC reflects the belief that internal factors and behaviors play a significant role in determining one’s health. CHLC involves the belief that health outcomes are determined by chance, luck, or fate. The MHLCS comprises 18 items, each rated on a six-point Likert scale ranging from “strongly disagree” (1) to “strongly agree” (6). Scores for each subscale range from 6 to 36, with higher scores indicating stronger beliefs in that particular locus of control. Each subscale is scored and estimated independently. The validity and reliability of the MHLCS were examined and confirmed by Moshki et al. in Iran in 2007. The reliability of the questionnaire was also confirmed by Cronbach’s α coefficients for the IHLC, CHLC, and PHLC subscales, which were 0.68, 0.66, and 0.72, respectively [32].
Perceived social support for breast cancer screening research-made questionnaire
The perceived social support for breast cancer screening questionnaire, developed by Bashirian et al. [1], was utilized. This questionnaire comprised items rated on a 5-point Likert scale, including five questions related to emotional support, three questions about informational support, and additional questions regarding instrumental and appraisal support. Face validity was established by collecting feedback from ten women regarding the simplicity, clarity, and readability of the questionnaire. The content validity of the questionnaire was confirmed by ten specialists in health education, gynecology, and oncology. Reliability was evaluated using Cronbach’s α coefficient and test re-test methods, yielding coefficients of 0.83 and 0.99, respectively [1]. The reliability of the social support questionnaire using Cronbach’s α coefficient was 0.958, which indicated the appropriate reliability. The questions were rated on a Likert scale from 1 to 5, with options ranging from “strongly disagree” to “strongly agree.” The score for each subscale and the total score were calculated by summing up the scores of the relevant questions and all items, respectively [33]. The current study categorized total scores into four groups: Very Low (scores of 14-28), low (scores of 29-42), moderate (scores of 43-56), and high (scores of 57-70) levels of perceived social support. This classification was based on the quartiles of the score distribution.
Statistical analysis
Data were analyzed using SPSS software, version 21.0 (SPSS Inc., Chicago, IL, USA). Only participants with complete data were included in the analysis. The normality of the distribution of quantitative variables was assessed using the Kolmogorov-Smirnov statistical test. Normal and non-normal quantitative variables were described using the Mean±SD and median (interquartile range), respectively. Qualitative variables were described using frequency (percentage). To investigate the relationship between health locus of control, perceived social support, and breast cancer screening behaviors, regression analysis was utilized to adjust for potential confounding variables. Variables that showed significance at P<0.2 in the simple regression models were incorporated into the multiple logistic regression models. Subsequently, variables with a P<0.05 in the multiple logistic regression model were considered significant.
Results
Demographic and individual characteristics of the participants
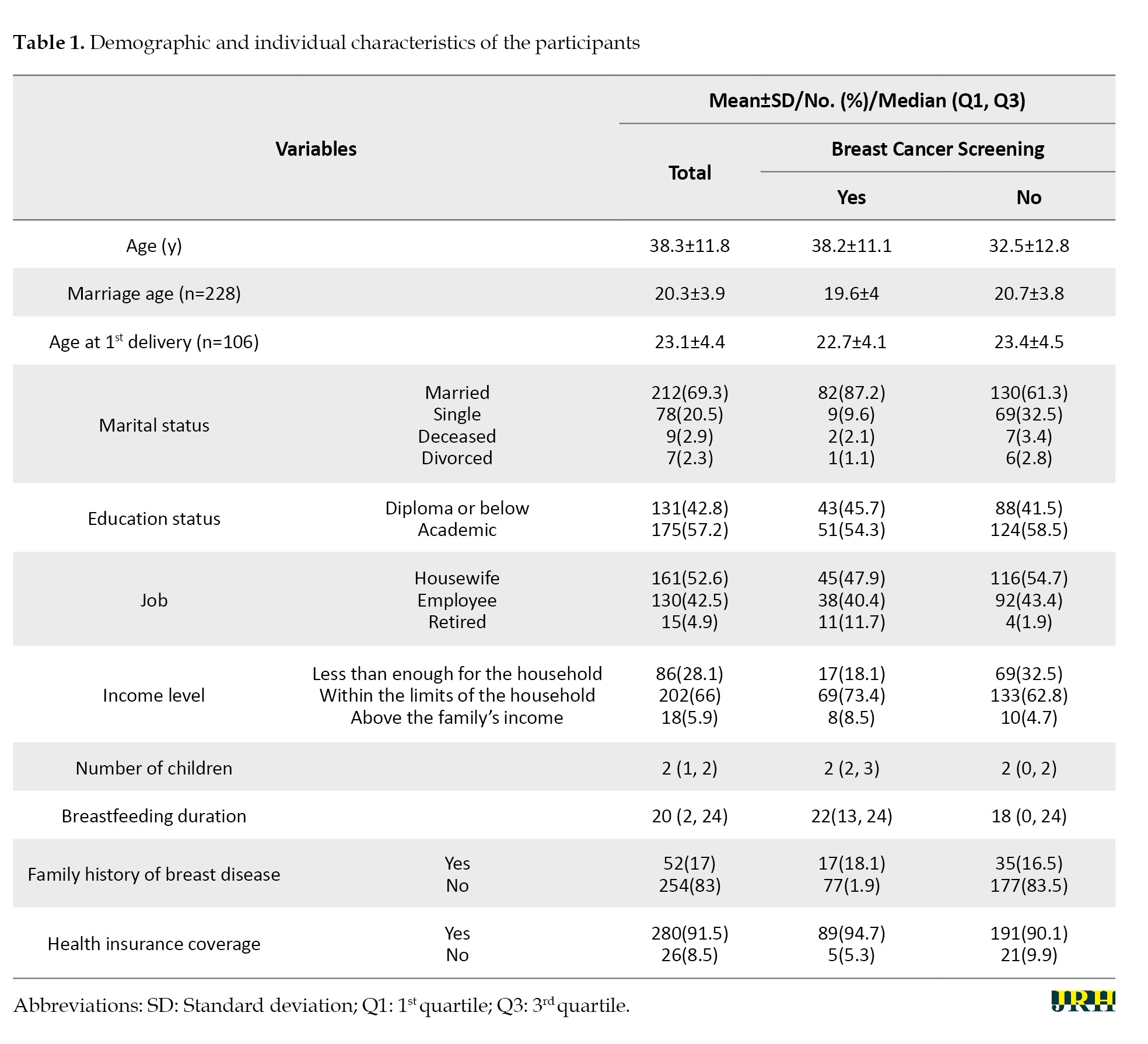
This study involved 311 participants; however, 5 individuals were excluded due to missing data, resulting in data analysis of 306 individuals. The mean age of the participants was 38.3±11.8 years, and 91.5% of the participants had health insurance coverage. Most participants (71.9%) reported that their income was adequate, and 57.2% had a university education. Also, 69.3% of the participants were married and 52.6% were housewives. In addition, 93.5% of them had no history of breast disease, and 17.0% had a family history of breast disease. Other demographic and personal characteristics of the participants are given in Table 1.
Breast cancer screening prevalence and knowledge levels among participants
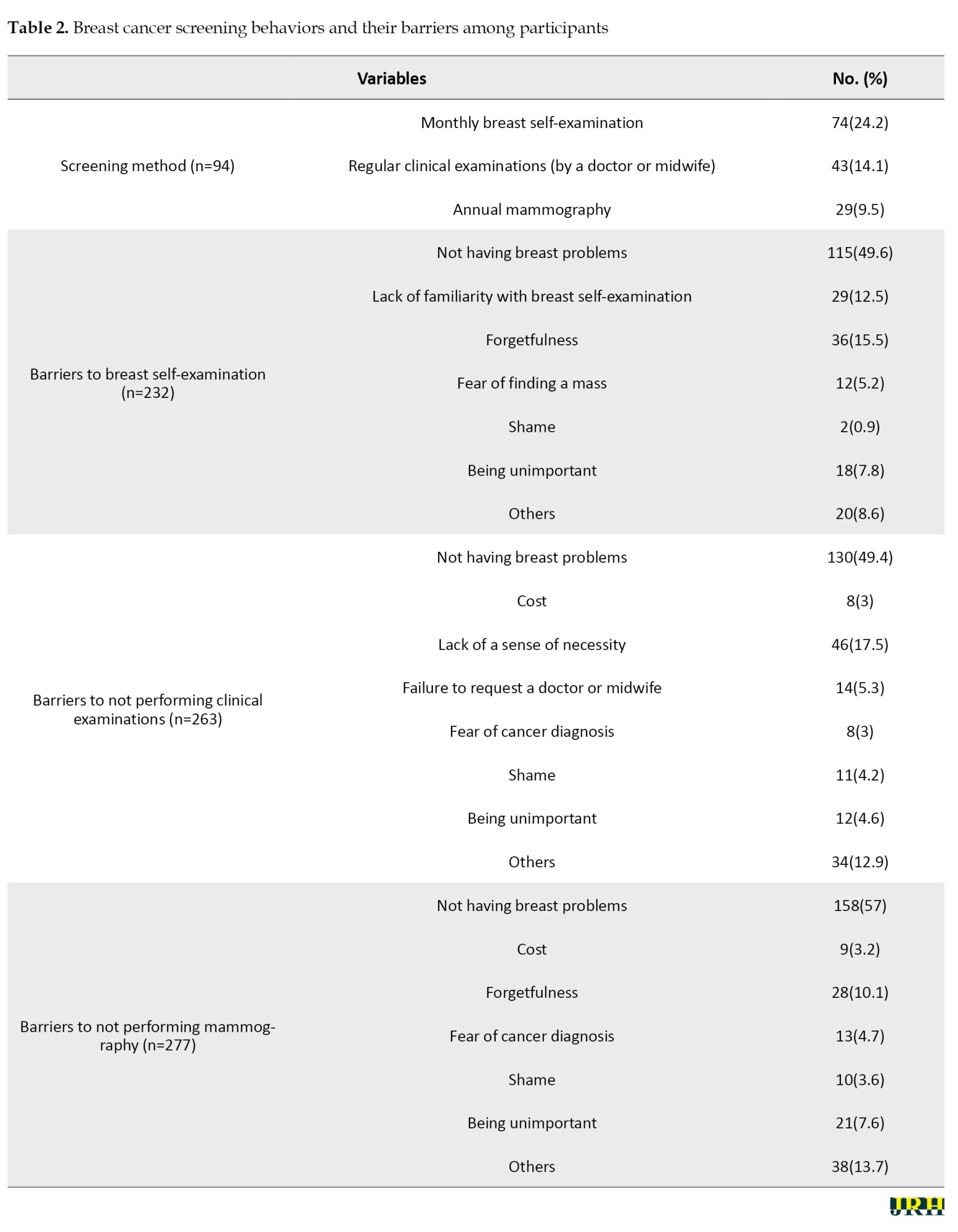
The overall prevalence of breast cancer screening was 30.7% (95% confidence interval (CI): 25.5%, 36.6%). The most common breast cancer screening method was monthly self-examination reported by 74 participants (24.2%), followed by regular clinical examinations by 43 participants (14.1%) and annual mammography by 29 participants (9.5%). Most of the participants (more than half) stated that not having a breast problem was the reason for not doing breast cancer screening. Other reasons for not performing breast cancer screening are given in Table 2.
The Mean±SD score of knowledge regarding breast cancer screening among the participants was 4.2±1.6. The knowledge level of 178(78.1%) participants was classified as abysmal, while 50 individuals (21.9%) had fair knowledge.
Health locus of control and perceived social support among participants
Table 3 shows the descriptive statistics of perceived health locus of control and social support among the participants.
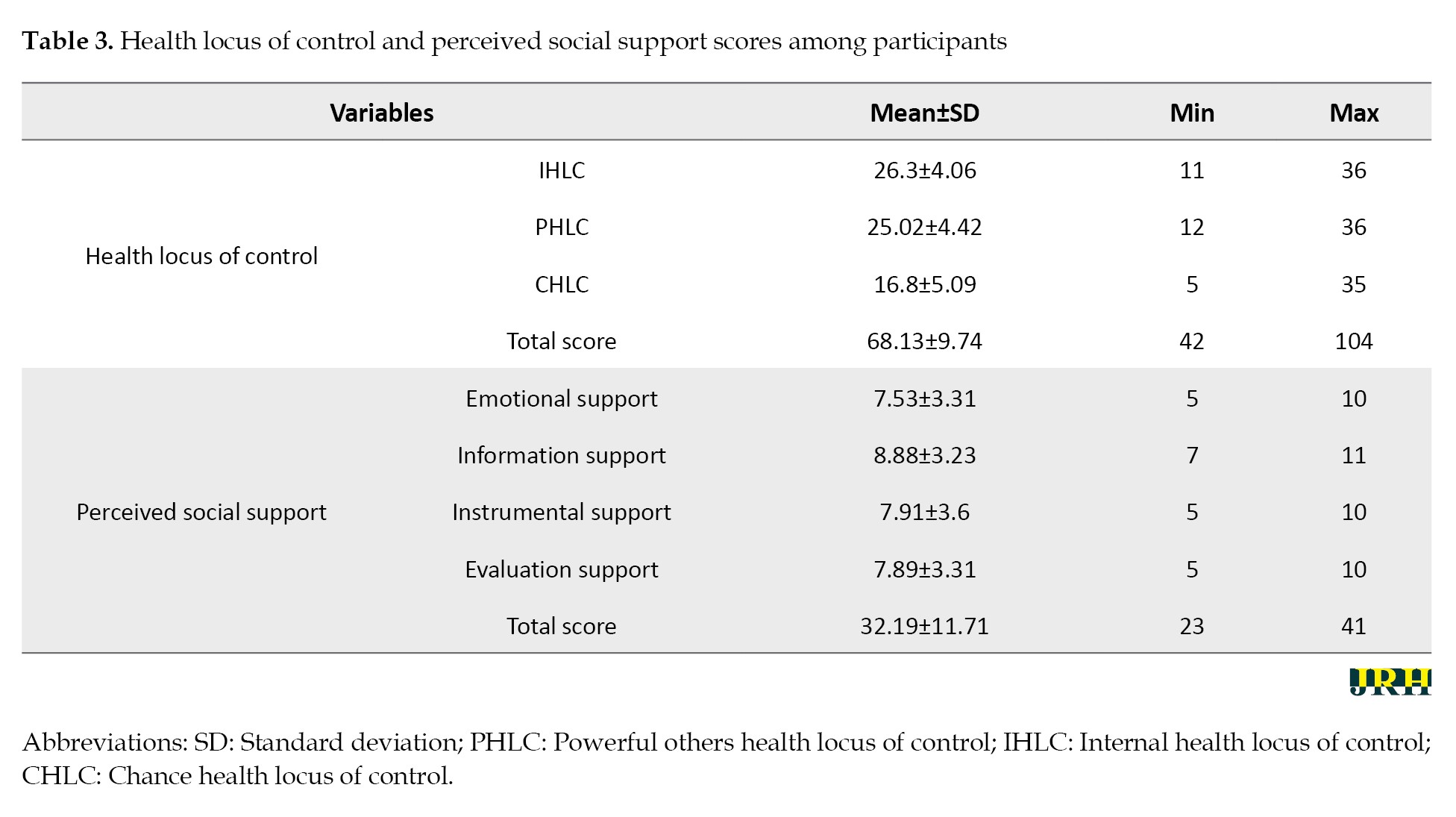
The highest average score was related to the internal health control source (Mean±SD 26.3±4.06), followed by the effective people’s health control source (Mean±SD 25.02±4.42). Among the components of social support, the highest score was related to emotional support (Mean±SD=26.3±4.06). The Mean±SD score of the perceived social support among the participants was 38.75±11.31. The study found that 39.0% of participants reported low or very low levels of perceived social support. Additionally, 41.5% reported moderate levels, while only 1.1% reported high levels of perceived social support (Table 3).
The role of health locus of control and perceived social support in breast cancer screening
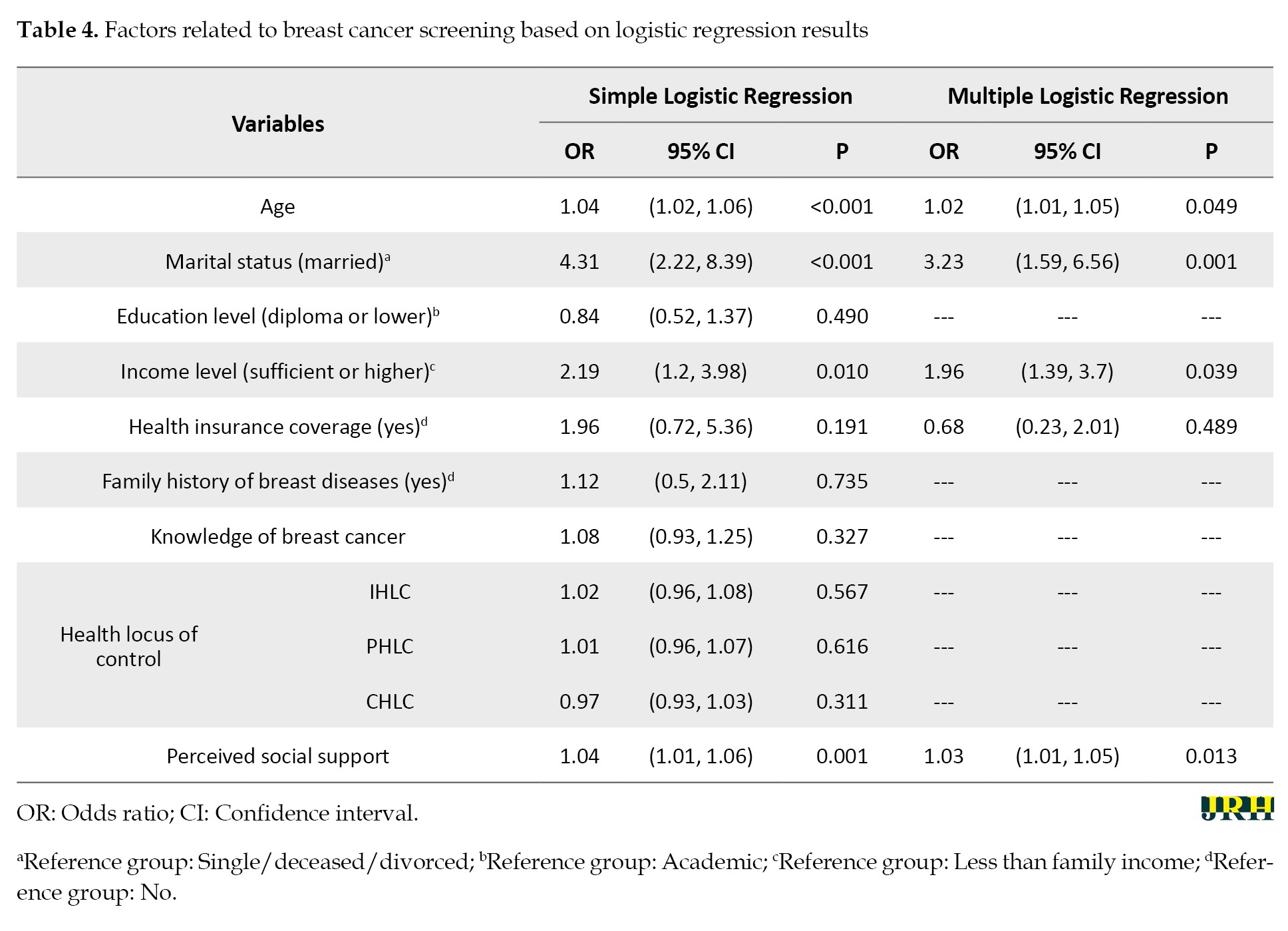
The variables of age, marital status, income level, and health insurance coverage were found to have P<0.2 in simple logistic regression, leading them to be included in the multiple logistic regression analysis. The results of the simple logistic regression model indicated a significant relationship between age, marital status, and income level and cancer screening. Specifically, older individuals (odds ratio (OR)=1.02, 95% CI, 1.01%, 1.05%, P=0.049), married individuals (OR=3.23, 95% CI, 1.59%, 6.56%, P=0.001), and those with higher income levels (OR=1.96, 95% CI, 1.03%, 3.7%, P=0.039) were more likely to undergo cancer screening.
The analysis revealed no significant relationship between health locus of control and cancer screening (P>0.05), while a significant association was found between perceived social support and cancer screening (P=0.001). The multiple logistic regression analysis revealed that the relationship between perceived social support and cancer screening remained significant, even after adjusting for confounding variables, such as age, marital status, income level, and health insurance coverage. Specifically, for each one-unit increase in the perceived probable support score, the odds of undergoing cancer screening increased between 1.01 and 1.05 times (OR=1.03, 95% CI, 1.01%, 1.05%, P=0.013) (Table 4).
Discussion
This cross-sectional study aimed to assess the relationship between breast cancer screening behaviors, the health locus of control, and social support among women in Gonabad, Iran.
The study found that a significant proportion of women had limited knowledge about breast cancer screening methods and that screening rates were low. These results are consistent with those of Mirzaei-Alavijeh et al. [34] and Mohaghegh et al. [35]. According to Noori et al. [36], one of the most important obstacles to breast cancer screening is a lack of awareness and knowledge. Therefore, it is necessary to hold educational programs regarding various breast cancer screening methods to improve women’s awareness. It seems that improving the awareness and attitude of women regarding the risk factors and signs and symptoms of breast cancer, and understanding the need for timely referral, plays an important role in increasing their participation in the breast cancer screening program.
In the current study, there was a statistically significant relationship between age, marital status, and income level and breast cancer screening, consistent with the results of Rejali et al. [37] and Saei Ghare Naz et al. [38] The results of Lam et al. [39] showed that there is a significant relationship between screening and women’s age, marital status, and employment. Regarding the considerable effect of age, it seems that increasing age is associated with greater health awareness, as the probability of developing breast cancer at an older age is higher. Consequently, women’s perceived threat may have increased, and having more time to participate in screening programs can also be among the reasons mentioned.
According to Mirzaei-Alavijeh et al. [34], the rates of breast self-examination, examination by a doctor, and mammography were 28%, 18%, and 17%, respectively, among individuals over 20 years old in Kermanshah. These rates are consistent with our results (24.2%, 14.1%, and 9.5%, respectively). The findings indicate low screening rates, highlighting the need for educational interventions to encourage women in this field. Also, according to Irani et al. [40], only 18.8% of women over the age of 20 performed breast self-examination, 19.1% had never performed a clinical breast examination, and 3.3% had a history of mammography. These statistics demonstrate the inadequate and weak performance of women regarding breast cancer screening. The most important reasons for not performing screening were mentioned as not having breast problems, which is consistent with the study by Rejali et al. [37].
Another factor in performing health behaviors is the health locus of control. People with a stronger internal belief in health control tend to engage in more health-promoting behaviors. In this study, no significant association was found between health control beliefs and participation in screening behaviors. Nowak et al. [41] investigated the screening rate among women with a positive family history. They showed no significant relationship between the health locus of control and screening. In the present study, the highest average score was related to the internal health locus of control, and the lowest was related to luck. Saei Ghare Naz et al. [42] also reported similar results. One of the reasons for the contradictory results of these studies is the existence of a different research community with diverse cultural and ethnic characteristics, which can affect the results.
Among the other findings of the current study, a statistically significant relationship between perceived social support and breast cancer screening was observed. Specifically, for each one-unit increase in the perceived social support score, the likelihood of undergoing breast cancer screening increased by a factor between 1.01 and 1.05. Emotional support received the highest score among the components of social support. In a systematic review by Hazavehei et al. [43], social support had a positive correlation with cancer screening behavior. Adegboyega et al. [23] showed that increased social support increased Pap smear screening among African immigrants, which is consistent with our study. In Pakistan, Saeed et al. [44] also reported the lack of awareness and perceived social support among people as the reason for not performing breast cancer screening.
Married individuals tend to exhibit increased screening behavior due to the support received from their spouses. Emotional support encompasses expressions of affection and love, care and reassurance, trust, sympathy, and attention. Perceived social support, as a suitable and accessible tool, can be used in intervention programs to enhance these behaviors. A strong sense of social support can help individuals feel more secure in their belief that they can rely on others for assistance when facing difficult situations, which may enable them to perceive potentially traumatic events as less stressful. When someone receives different treatment or opportunities based on their race, gender, or any other personal characteristic, it is referred to as discrimination. Individuals can receive various forms of social support to address and mitigate the adverse impacts of stress, which can impact self-confidence in facing illnesses [45].
Implications for clinicians and policymakers
The comprehensive health services centers in Iran’s primary healthcare system, “Irapen,” implement a set of basic interventions for non-communicable diseases, including the prevention and early diagnosis of breast cancer. As part of this initiative, all women aged 30 to 69 are encouraged to undergo breast cancer evaluation. This project aims to enhance awareness and understanding of breast cancer in women, ultimately encouraging more individuals to participate in screening. Additionally, health personnel play a crucial role in promoting screening behavior by advising on the importance of breast cancer screening, referring high-risk individuals, and conducting training sessions.
Conclusion
The findings of this study indicate that low levels of knowledge and infrequent breast cancer screening behaviors, combined with the influence of social support, suggest that promoting screening could be an effective strategy for preventing breast cancer in women. Consequently, it is recommended that targeted educational interventions be developed and implemented, taking into account the role of social support in encouraging screening behaviors.
Limitations of the study
Due to its design, the current study is limited by self-reported bias and cannot establish causal relationships. Additionally, the findings may not be generalizable to all regions of Iran. Conducting population-based studies is advised to enhance the efficacy of breast cancer screening programs on a national scale.
Ethical Considerations
Compliance with ethical guidelines
This study is based on a research project approved by the Ethics Committee of Gonabad University of Medical Sciences, Gonabad, Iran (Code: IR.GMU.REC.1400.215). All procedures performed in this study adhered to the ethical standards of the institutional and/or national research committee, as well as the 1964 Helsinki Declaration and its later amendments or comparable standards. Written informed consent was obtained from all subjects.
Funding
This study was financially supported by the Social Development and Health Promotion Research Center, Gonabad University of Medical Sciences, Gonabad, Iran.
Authors' contributions
Conceptualization and study design: Mitra Dogonchi, Mahdi Moshki, and Fatemeh Mohammadzadeh; Data collection: Elham Saberi Noghabi; Data interpretation and analysis: Fatemeh Mohammadzadeh; Investigation: Mitra Dogonchi; Writing: Mitra Dogonchi and Fatemeh Mohammadzadeh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Social Development and Health Promotion Research Center at Gonabad University of Medical Sciences, as well as all those who assisted the authors in conducting this research project.
References
Breast cancer is the most common malignancy known in the world [1]. The projections from global burden of breast cancer surveillance studies indicate that the number of new cases is expected to increase by 40%, reaching more than three million. In addition, breast cancer deaths will increase by 50% to one million by 2040 [2]. In Iran, breast cancer ranks first in terms of incidence and fifth in terms of mortality, accounting for 13% of all cancers regardless of gender, with an age-standardized incidence rate (ASIR) of 35.8 and an age-standardized mortality rate (ASMR) of 10.8 per 100,000 women, which is currently lower than neighboring countries with similar income levels. This rate has increased compared to previous reports; thus, in the latest published report of Iran’s national cancer registration program, the incidence rate was 34.5 per 100,000 women [3, 4]. Currently, the incidence of this cancer is increasing in Iran, and unfortunately, women in Iran are at advanced stages of breast cancer at a young age [3].
If this cancer is detected in the early stages using screening methods, it can be treated [5]. Screening methods for the early diagnosis of this deadly disease include mammography, monthly self-examination, and clinical examinations [6]. Mammography is recommended for women over 40 years old, while clinical breast examinations should be conducted every three years for women between 20 and 40 years old, and annually by a specialist for those over 40. Additionally, breast self-examination is advised for women over 20 years old on a monthly basis [7]. Studies have revealed that people have a low level of awareness regarding breast cancer screening coverage. When women are more aware of breast cancer, they are more likely to undergo screening, which can have a positive impact on their health. Awareness refers to the ability to recall details, understand general procedures, recognize processes, identify patterns, and comprehend structures or situations related to breast cancer screening [8].
Promoting preventive behaviors will improve people’s performance, increase their quality of life, and reduce healthcare costs [9]. Research has found that improving knowledge, social support, and a high health locus of control can encourage healthy behaviors [10-12]. One factor that promotes health-related behaviors in a person is the center of health control [13]. Health locus of control is the extent of a person’s control over certain events in his/her life, which ultimately predicts health behavior based on people’s beliefs [14]. These factors include the following:
The internal health locus of control (IHLC) encompasses the degree to which a person believes that their internal factors [15] and behaviors are responsible for their health and illness. The control axis of effective people includes the extent to which a person believes that other individuals determine his/her health. The axis of luck control includes the degree of a person’s belief that his/her health depends on his/her luck, fortune, and destiny [15]. People who rely more on themselves have poorer cooperation with healthcare providers. The concept examined through the health locus of control is the perception of personal effectiveness and individual responsibility regarding health [16]. The health locus of control has been shown to be positively related to engagement in health behaviors [17]. Understanding the mechanisms of this relationship could help identify groups that may benefit from targeting certain mediators. Social support could mediate the relationship between IHLC and health behaviors, such as physical activity, dietary practices, and screening [18]. The health locus of control is associated with better health behaviors through the mediating effect of social support. The persuasion and motivation of those around individuals with higher IHLC positively affected their ability to engage in certain health behaviors [19, 20]. In addition, the results of other studies show that social support also plays a key role in performing health behaviors and screening [16, 21-23]. Social support provides access to information, encourages people, and helps them adopts preventive behaviors. Social support through increasing self-efficacy leads to overcoming perceived obstacles (emotional, logical, and financial) in breast cancer screening [24, 25]. Social support refers to the awareness that a person is part of a society that loves and values him/her. Social support includes tangible components, such as financial and physical assistance, and intangible elements, such as encouragement and guidance. This support can have different forms. There are four main types of social support. Emotional support includes offering sympathy, love, trust, and care. Instrumental support means a tangible help that a person needs. Informational support means providing advice, comments, and information that a person can use when facing a problem. Evaluation support involves providing valuable information for self-assessment [26].
Given that health control beliefs and perceived social support play an important role in health behaviors [27], and considering the growing trend of breast cancer in Iran, along with the referral of many breast cancer patients in advanced stages of the disease, it is crucial to reflect on and address this issue to promote behaviors that lead to the early diagnosis of breast cancer in order to reduce mortality associated with it [28]. Therefore, the present study aimed to investigate the role of health control beliefs and perceived social support in breast cancer screening in women covered by comprehensive health service centers in Gonabad (Northeast Iran) in 2022.
Methods
Design, settings, and participants
This cross-sectional study was conducted on women referred by the comprehensive health service centers in Gonabad, located in northeastern Iran, from February 2022 to March 2023. The inclusion criteria were being 20 years or older and providing informed consent to participate, with no physical or mental problems, and no history of breast self-examination in the study. Women with current or suspected diagnoses of breast cancer or those with incomplete questionnaires were excluded from the study. Based on the sample size formula (Equation 1):
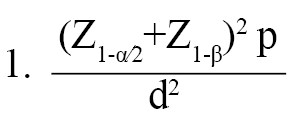
, a type 1 error (α) of 0.05, a test power (β) of 0.80, and an error (d) of 0.05, the sample size required to estimate the prevalence of breast cancer screening in Iranian women, based on previous studies [27-29] suggesting a 10% prevalence, was initially calculated to be 283 samples. To account for a potential 10% drop in participation, the sample size was increased to 311. This sample size was deemed adequate for logistic regression analysis based on the rule of events per variable (EPV). It is recommended that an EPV of 10 is acceptable for logistic regression [30].
The sampling method was a stratified random sample proportional to the population size. In this approach, each of the comprehensive health service centers in Gonabad City was considered a stratum, and samples were selected through simple random sampling from the list of women aged 20 and above in each center, proportional to the population served and the determined sample size.
Instruments
Data were collected using self-administered questionnaires.
Demographic checklist
It included several items on age, marital age, age at first delivery, marital status, education level, job, income level, number of children, breastfeeding duration, health insurance coverage, family history of breast disease, and some items about breast cancer screening behaviors and their barriers.
Breast cancer knowledge research-made questionnaire
This questionnaire included questions categorized under three main topics: Breast cancer potential risk factors, signs and symptoms, and cancer screening and prevention. To ensure the questionnaire’s validity, a committee of experts in research methodology, obstetrics and gynecology, and oncology reviewed and approved the questions. A pilot study involving 30 participants was conducted to test the questionnaire’s clarity and reliability. The reliability of the questionnaire, measured using the Kuder-Richardson coefficient, was 0.705. Respondents were asked to select their answers from ‘yes’, ‘no’, or ‘don’t know’. These responses were then dichotomized, with correct answers receiving a score of 1 and ‘don’t know’ or incorrect answers receiving a score of 0. Each participant’s total knowledge score was calculated by summing their responses, with a maximum possible score of 10.
Based on the quartiles, participants’ total knowledge scores were categorized as follows: inferior knowledge (score of 0–2.5), poor knowledge (score of 2.6–5), fair knowledge (score of 5.1–7.5), and good knowledge (score of 7.5–10).
Breast cancer screening behaviors and their barriers research-made questionnaire
This scale included six items concerning breast cancer screening methods (self-examination, clinical examinations, and mammography) and barriers to women’s breast cancer screening. For example, it asked, “Do you perform breast self-examination monthly? If no, what obstacles do you face when it comes to performing breast self-examinations?” The reliability of this questionnaire, measured using the Kuder-Richardson coefficient, was 0.705.
Multidimensional health locus of control scale (MHLCS)
The MHLCS was developed in 1987 by Wallston et al. [31] to assess individuals’ beliefs about their control over their health. This questionnaire measures three main domains: Powerful others health locus of control (PHLC), IHLC, and chance health locus of control (CHLC). PHLC refers to the belief that an individual’s health is affected by external factors. IHLC reflects the belief that internal factors and behaviors play a significant role in determining one’s health. CHLC involves the belief that health outcomes are determined by chance, luck, or fate. The MHLCS comprises 18 items, each rated on a six-point Likert scale ranging from “strongly disagree” (1) to “strongly agree” (6). Scores for each subscale range from 6 to 36, with higher scores indicating stronger beliefs in that particular locus of control. Each subscale is scored and estimated independently. The validity and reliability of the MHLCS were examined and confirmed by Moshki et al. in Iran in 2007. The reliability of the questionnaire was also confirmed by Cronbach’s α coefficients for the IHLC, CHLC, and PHLC subscales, which were 0.68, 0.66, and 0.72, respectively [32].
Perceived social support for breast cancer screening research-made questionnaire
The perceived social support for breast cancer screening questionnaire, developed by Bashirian et al. [1], was utilized. This questionnaire comprised items rated on a 5-point Likert scale, including five questions related to emotional support, three questions about informational support, and additional questions regarding instrumental and appraisal support. Face validity was established by collecting feedback from ten women regarding the simplicity, clarity, and readability of the questionnaire. The content validity of the questionnaire was confirmed by ten specialists in health education, gynecology, and oncology. Reliability was evaluated using Cronbach’s α coefficient and test re-test methods, yielding coefficients of 0.83 and 0.99, respectively [1]. The reliability of the social support questionnaire using Cronbach’s α coefficient was 0.958, which indicated the appropriate reliability. The questions were rated on a Likert scale from 1 to 5, with options ranging from “strongly disagree” to “strongly agree.” The score for each subscale and the total score were calculated by summing up the scores of the relevant questions and all items, respectively [33]. The current study categorized total scores into four groups: Very Low (scores of 14-28), low (scores of 29-42), moderate (scores of 43-56), and high (scores of 57-70) levels of perceived social support. This classification was based on the quartiles of the score distribution.
Statistical analysis
Data were analyzed using SPSS software, version 21.0 (SPSS Inc., Chicago, IL, USA). Only participants with complete data were included in the analysis. The normality of the distribution of quantitative variables was assessed using the Kolmogorov-Smirnov statistical test. Normal and non-normal quantitative variables were described using the Mean±SD and median (interquartile range), respectively. Qualitative variables were described using frequency (percentage). To investigate the relationship between health locus of control, perceived social support, and breast cancer screening behaviors, regression analysis was utilized to adjust for potential confounding variables. Variables that showed significance at P<0.2 in the simple regression models were incorporated into the multiple logistic regression models. Subsequently, variables with a P<0.05 in the multiple logistic regression model were considered significant.
Results
Demographic and individual characteristics of the participants
This study involved 311 participants; however, 5 individuals were excluded due to missing data, resulting in data analysis of 306 individuals. The mean age of the participants was 38.3±11.8 years, and 91.5% of the participants had health insurance coverage. Most participants (71.9%) reported that their income was adequate, and 57.2% had a university education. Also, 69.3% of the participants were married and 52.6% were housewives. In addition, 93.5% of them had no history of breast disease, and 17.0% had a family history of breast disease. Other demographic and personal characteristics of the participants are given in Table 1.

Breast cancer screening prevalence and knowledge levels among participants
The overall prevalence of breast cancer screening was 30.7% (95% confidence interval (CI): 25.5%, 36.6%). The most common breast cancer screening method was monthly self-examination reported by 74 participants (24.2%), followed by regular clinical examinations by 43 participants (14.1%) and annual mammography by 29 participants (9.5%). Most of the participants (more than half) stated that not having a breast problem was the reason for not doing breast cancer screening. Other reasons for not performing breast cancer screening are given in Table 2.

The Mean±SD score of knowledge regarding breast cancer screening among the participants was 4.2±1.6. The knowledge level of 178(78.1%) participants was classified as abysmal, while 50 individuals (21.9%) had fair knowledge.
Health locus of control and perceived social support among participants
Table 3 shows the descriptive statistics of perceived health locus of control and social support among the participants.

The highest average score was related to the internal health control source (Mean±SD 26.3±4.06), followed by the effective people’s health control source (Mean±SD 25.02±4.42). Among the components of social support, the highest score was related to emotional support (Mean±SD=26.3±4.06). The Mean±SD score of the perceived social support among the participants was 38.75±11.31. The study found that 39.0% of participants reported low or very low levels of perceived social support. Additionally, 41.5% reported moderate levels, while only 1.1% reported high levels of perceived social support (Table 3).
The role of health locus of control and perceived social support in breast cancer screening
The variables of age, marital status, income level, and health insurance coverage were found to have P<0.2 in simple logistic regression, leading them to be included in the multiple logistic regression analysis. The results of the simple logistic regression model indicated a significant relationship between age, marital status, and income level and cancer screening. Specifically, older individuals (odds ratio (OR)=1.02, 95% CI, 1.01%, 1.05%, P=0.049), married individuals (OR=3.23, 95% CI, 1.59%, 6.56%, P=0.001), and those with higher income levels (OR=1.96, 95% CI, 1.03%, 3.7%, P=0.039) were more likely to undergo cancer screening.
The analysis revealed no significant relationship between health locus of control and cancer screening (P>0.05), while a significant association was found between perceived social support and cancer screening (P=0.001). The multiple logistic regression analysis revealed that the relationship between perceived social support and cancer screening remained significant, even after adjusting for confounding variables, such as age, marital status, income level, and health insurance coverage. Specifically, for each one-unit increase in the perceived probable support score, the odds of undergoing cancer screening increased between 1.01 and 1.05 times (OR=1.03, 95% CI, 1.01%, 1.05%, P=0.013) (Table 4).

Discussion
This cross-sectional study aimed to assess the relationship between breast cancer screening behaviors, the health locus of control, and social support among women in Gonabad, Iran.
The study found that a significant proportion of women had limited knowledge about breast cancer screening methods and that screening rates were low. These results are consistent with those of Mirzaei-Alavijeh et al. [34] and Mohaghegh et al. [35]. According to Noori et al. [36], one of the most important obstacles to breast cancer screening is a lack of awareness and knowledge. Therefore, it is necessary to hold educational programs regarding various breast cancer screening methods to improve women’s awareness. It seems that improving the awareness and attitude of women regarding the risk factors and signs and symptoms of breast cancer, and understanding the need for timely referral, plays an important role in increasing their participation in the breast cancer screening program.
In the current study, there was a statistically significant relationship between age, marital status, and income level and breast cancer screening, consistent with the results of Rejali et al. [37] and Saei Ghare Naz et al. [38] The results of Lam et al. [39] showed that there is a significant relationship between screening and women’s age, marital status, and employment. Regarding the considerable effect of age, it seems that increasing age is associated with greater health awareness, as the probability of developing breast cancer at an older age is higher. Consequently, women’s perceived threat may have increased, and having more time to participate in screening programs can also be among the reasons mentioned.
According to Mirzaei-Alavijeh et al. [34], the rates of breast self-examination, examination by a doctor, and mammography were 28%, 18%, and 17%, respectively, among individuals over 20 years old in Kermanshah. These rates are consistent with our results (24.2%, 14.1%, and 9.5%, respectively). The findings indicate low screening rates, highlighting the need for educational interventions to encourage women in this field. Also, according to Irani et al. [40], only 18.8% of women over the age of 20 performed breast self-examination, 19.1% had never performed a clinical breast examination, and 3.3% had a history of mammography. These statistics demonstrate the inadequate and weak performance of women regarding breast cancer screening. The most important reasons for not performing screening were mentioned as not having breast problems, which is consistent with the study by Rejali et al. [37].
Another factor in performing health behaviors is the health locus of control. People with a stronger internal belief in health control tend to engage in more health-promoting behaviors. In this study, no significant association was found between health control beliefs and participation in screening behaviors. Nowak et al. [41] investigated the screening rate among women with a positive family history. They showed no significant relationship between the health locus of control and screening. In the present study, the highest average score was related to the internal health locus of control, and the lowest was related to luck. Saei Ghare Naz et al. [42] also reported similar results. One of the reasons for the contradictory results of these studies is the existence of a different research community with diverse cultural and ethnic characteristics, which can affect the results.
Among the other findings of the current study, a statistically significant relationship between perceived social support and breast cancer screening was observed. Specifically, for each one-unit increase in the perceived social support score, the likelihood of undergoing breast cancer screening increased by a factor between 1.01 and 1.05. Emotional support received the highest score among the components of social support. In a systematic review by Hazavehei et al. [43], social support had a positive correlation with cancer screening behavior. Adegboyega et al. [23] showed that increased social support increased Pap smear screening among African immigrants, which is consistent with our study. In Pakistan, Saeed et al. [44] also reported the lack of awareness and perceived social support among people as the reason for not performing breast cancer screening.
Married individuals tend to exhibit increased screening behavior due to the support received from their spouses. Emotional support encompasses expressions of affection and love, care and reassurance, trust, sympathy, and attention. Perceived social support, as a suitable and accessible tool, can be used in intervention programs to enhance these behaviors. A strong sense of social support can help individuals feel more secure in their belief that they can rely on others for assistance when facing difficult situations, which may enable them to perceive potentially traumatic events as less stressful. When someone receives different treatment or opportunities based on their race, gender, or any other personal characteristic, it is referred to as discrimination. Individuals can receive various forms of social support to address and mitigate the adverse impacts of stress, which can impact self-confidence in facing illnesses [45].
Implications for clinicians and policymakers
The comprehensive health services centers in Iran’s primary healthcare system, “Irapen,” implement a set of basic interventions for non-communicable diseases, including the prevention and early diagnosis of breast cancer. As part of this initiative, all women aged 30 to 69 are encouraged to undergo breast cancer evaluation. This project aims to enhance awareness and understanding of breast cancer in women, ultimately encouraging more individuals to participate in screening. Additionally, health personnel play a crucial role in promoting screening behavior by advising on the importance of breast cancer screening, referring high-risk individuals, and conducting training sessions.
Conclusion
The findings of this study indicate that low levels of knowledge and infrequent breast cancer screening behaviors, combined with the influence of social support, suggest that promoting screening could be an effective strategy for preventing breast cancer in women. Consequently, it is recommended that targeted educational interventions be developed and implemented, taking into account the role of social support in encouraging screening behaviors.
Limitations of the study
Due to its design, the current study is limited by self-reported bias and cannot establish causal relationships. Additionally, the findings may not be generalizable to all regions of Iran. Conducting population-based studies is advised to enhance the efficacy of breast cancer screening programs on a national scale.
Ethical Considerations
Compliance with ethical guidelines
This study is based on a research project approved by the Ethics Committee of Gonabad University of Medical Sciences, Gonabad, Iran (Code: IR.GMU.REC.1400.215). All procedures performed in this study adhered to the ethical standards of the institutional and/or national research committee, as well as the 1964 Helsinki Declaration and its later amendments or comparable standards. Written informed consent was obtained from all subjects.
Funding
This study was financially supported by the Social Development and Health Promotion Research Center, Gonabad University of Medical Sciences, Gonabad, Iran.
Authors' contributions
Conceptualization and study design: Mitra Dogonchi, Mahdi Moshki, and Fatemeh Mohammadzadeh; Data collection: Elham Saberi Noghabi; Data interpretation and analysis: Fatemeh Mohammadzadeh; Investigation: Mitra Dogonchi; Writing: Mitra Dogonchi and Fatemeh Mohammadzadeh; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Social Development and Health Promotion Research Center at Gonabad University of Medical Sciences, as well as all those who assisted the authors in conducting this research project.
References
- Bashirian S, Barati M, Mohammadi Y, MoaddabShoar L, Dogonchi M. Evaluation of an intervention program for promoting breast self-examination behavior in employed women in Iran. Breast Cancer: Basic and Clinical Research 2021, 15:1178223421989657. [DOI:10.1177/1178223421989657] [PMID]
- Sanaat Z, Dolatkhah R. Epidemiologic profile of breast cancer in Iran: A systematic review and meta-analysis. Clinical Epidemiology and Global Health. 2024; 26:101537. [DOI:10.1016/j.cegh.2024.101537]
- Dolatkhah R, Hosseinalifam M, Sanaat Z, Dolatkhah N, Dastgiri S. Molecular Epidemiology of Breast Cancer in Iran: A Review Article. Journal of Obstetrics, Gynecology and Cancer Research. 2023, 8(5):422-30. [DOI:10.30699/jogcr.8.5.422]
- Haghighat S, Omidi Z, Ghanbari-Motlagh A. Trend of breast cancer incidence in Iran during a fifteen-year interval according to national cancer registry reports. Iranian Journal of Breast Diseases. 2022, 15(2):4-17. [DOI:10.30699/ijbd.15.2.4]
- Ginsburg O, Yip CH, Brooks A, Cabanes A, Caleffi M, Dunstan Yataco JA, et al. Breast cancer early detection: A phased approach to implementation. Cancer. 2020; 126(Suppl 10):2379-93. [DOI:10.1002/cncr.32887] [PMID]
- Bhan AD, Jayaram J. Screening, self-examination and awareness in breast cancer. In: Sharma SC, Mazumdar A, Kaushik R, editors. Breast Cancer. Singapore: Springer Nature Singapore; 2022. [DOI:10.1007/978-981-16-4546-4_29]
- Schünemann HJ, Lerda D, Quinn C, Follmann M, Alonso-Coello P, Rossi PG, et al. Breast cancer screening and diagnosis: A synopsis of the European breast guidelines. Annals of Internal Medicine 2020; 172(1):46-56. [DOI:10.7326/M19-2125] [PMID]
- Mohebi Z, Heidari Sarvestani M, Moradi Z, Naghizadeh MM. Female high school students’ knowledge and attitude toward breast cancer. BMC Women’s Health. 2023; 23(1):41. [DOI:10.1186/s12905-023-02155-z] [PMID]
- Hutchcraft ML, Teferra AA, Montemorano L, Patterson JG. Differences in health-related quality of life and health behaviors among lesbian, bisexual, and heterosexual women surviving cancer from the 2013 to 2018 National Health Interview Survey. LGBT Health. 2021; 8(1):68-78. [DOI:10.1089/lgbt.2020.0185] [PMID]
- Mishra SI, DeForge B, Barnet B, Ntiri S, Grant L. Social determinants of breast cancer screening in urban primary care practices: A community-engaged formative study. Women’s Health Issues. 2012; 22(5):e429-38. [DOI:10.1016/j.whi.2012.06.004] [PMID]
- Saei Ghare Naz M, Darooneh T, Salmani F, Kholosi Badr, Ozgoli G. Relationship of Health Locus of Control with Breast Cancer Screening Belief of Iranian Women. Asian Pacific Journal of Cancer Prevention. 2019; 20(3):699-703. [DOI:10.31557/APJCP.2019.20.3.699] [PMID]
- Williams-Piehota P, Schneider TR, Pizarro J, Mowad L, Salovey P. Matching health messages to health locus of control beliefs for promoting mammography utilization. Psychology & Health. 2004; 19(4):407-23. [DOI:10.1080/08870440310001652678]
- Fathabadi J, Sadeghi S, Jomhari F, Talaneshan A. [The role of health-oriented lifestyle and health locus of control in predicting the risk of overweight (Persian)]. Iranian Journal of Health Education and Health Promotion. 2018; 5(4):280-7. [DOI:10.30699/acadpub.ijhehp.5.4.280]
- Kesavayuth D, Poyago-Theotoky J, Zikos V. Locus of control, health and healthcare utilization. Economic Modelling. 2020; 86:227-38. [DOI:10.1016/j.econmod.2019.06.014]
- Olagoke AA, Olagoke OO, Hughes AM. Intention to vaccinate against the novel 2019 coronavirus disease: The role of health locus of control and religiosity. Journal of Religion and Health. 2021; 60(1):65-80. [DOI:10.1007/s10943-020-01090-9] [PMID]
- Hairaty K, Sadeghmoghadam L, Alami A, Moshki M. [Effect of education based on health locus of control theory on health literacy among older adults (Persian)]. Medical of Horizo. 2019; 25(1):37-42. [Link]
- VanderZee KI, Buunk BP, Sanderman R:. Social support, locus of control, and psychological well‐being. Journal of Applied Social Psychology. 1997; 27(20):1842-59. [DOI:10.1111/j.1559-1816.1997.tb01628.x]
- Lieber SB, Moxley J, Mandl LA, Reid MC, Czaja SJ. Social support and physical activity: Does general health matter? European Review of Aging and Physical Activity. 2024; 21(1):16. [DOI:10.1186/s11556-024-00347-6] [PMID]
- Marr J, Wilcox S. Self-efficacy and social support mediate the relationship between internal health locus of control and health behaviors in college students. American Journal of Health Education 2015, 46(3):122-31. [DOI:10.1080/19325037.2015.1023477]
- Kim J, Kwon M, Jung S. The influence of health locus of control, social support, and self-efficacy on health promoting behavior in middle-aged adults. Journal of the Korea Academia-Industrial cooperation Society. 2017; 18(4):494-503.[Link]
- Tahergorabi Z, Mohammadifard M, Salmani F, Moodi M. Breast cancer screening behavior and its associated factors in female employees in South Khorasan. Journal of Education and Health Promotion. 2021; 10:102. [DOI:10.4103/jehp.jehp_750_20] [PMID]
- Kugbey N, Oppong Asante K, Meyer-Weitz A. Depression, anxiety and quality of life among women living with breast cancer in Ghana: Mediating roles of social support and religiosity. Supportive Care in Cancer. 2020; 28(6):2581-8. [DOI:10.1007/s00520-019-05027-1] [PMID]
- Adegboyega A, Aroh A, Williams LB, Mudd-Martin G. Social support and cervical cancer screening among sub-Saharan African immigrant (SAI) women. Cancer Causes & Control. 2022; 33(6):823-30. [DOI:10.1007/s10552-022-01577-8] [PMID]
- Fayazi F, Araban M, Haghighi Zadeh MH, Mohamadian H. [Development and psychometric evaluation of a colorectal cancer screening scale based on preventive health model: Application of Smart-PLS software (Persian)]. Payesh (Health Monitor) 2019; 18(3):251-9. [Link]
- Çınar İÖ, Tuzcu A. Comparison of the levels of fear and perceived social support among the women having and not having mammography. Erciyes Medical Journal. 2020; 42(3). [Link]
- Ginja S, Coad J, Bailey E, Kendall S, Goodenough T, Nightingale S, et al. Associations between social support, mental wellbeing, self-efficacy and technology use in first-time antenatal women: Data from the BaBBLeS cohort study. BMC Pregnancy and Childbirth 2018; 18(1):441. [DOI:10.1186/s12884-018-2049-x] [PMID]
- Mahmoudabadi M, Saeidifar A, Safizade H. Breast cancer screening behavior among nurses in Kerman teaching hospitals and its relationship with the health beliefs model scales. Iranian Journal of Breast Diseases. 2018; 11(2):56-65. [DOI:10.30699/acadpub.ijbd.11.2.56]
- Enjezab B. Cancer screening practice among Iranian middle-aged women. Journal of Midwifery and Reproductive Health. 2016; 4(4):770-8. [Link]
- Rezabeigi-Davarani E, Khanjani N, Falahi M, Daneshi S, Iranpour A. Breast self-examination and its effective factors based on the theory of planned behavior among women in Kerman, Iran. Journal of Education and Community Health. 2016; 3(3):1-8. [DOI:10.21859/jech-03031]
- Bujang MA, Sa’at N, Bakar TMITA, Joo LC. Sample size guidelines for logistic regression from observational studies with large population: Emphasis on the accuracy between statistics and parameters based on real life clinical data. The Malaysian Journal of Medical Sciences: MJMS. 2018; 25(4):122-30. [DOI:10.21315/mjms2018.25.4.12] [PMID]
- Wallston KA, Strudler Wallston B, DeVellis R. Development of the multidimensional health locus of control (MHLC) scales. Health Education Monographs. 1978; 6(1):160-70. [DOI:10.1177/109019817800600107] [PMID]
- Moshki M, Ghofranipour F, Hajizadeh E, Azadfallah P. Validity and reliability of the multidimensional health locus of control scale for college students. BMC Public health. 2007; 7:295. [DOI:10.1186/1471-2458-7-295] [PMID]
- Mallery P, George D. SPSS for windows step by step. Massachusetts: Allyn & Bacon, Inc; 2000. [Link]
- Mirzaei-Alavijeh M, Amini M, Keshavarzi A, Jalilian F. [Inequality in breast cancer screening tests uptake among women in western Iran (Persian)]. Iranian Journal of Breast Diseases. 2023, 15(4):89-104. [DOI:10.30699/ijbd.15.4.89]
- Mohaghegh P, Farahani M, Moslemi A, Ahmadi F, Nazari J. [Participation rate, family histories, symptoms, and incidence of breast cancer in the screening program for breast cancer in the population covered by Arak health centers (Persian)]. Iranian Journal of Breast Diseases. 2021; 14(2):41-9. [DOI:10.30699/ijbd.14.2.41]
- Noori K, Sahraee P, Keshavarz Mohammadi N. [Barriers to participation of breast cancer patients’ relatives in mammographic screening (Persian)]. Iranian Journal of Breast Diseases. 2021; 14(2):26-40. [DOI:10.30699/ijbd.14.2.26]
- Rejali M, Yadegarfar G, Mostajeran M, Aghdak P, Fadaei R, Ansari R. [Evaluation of the status of breast cancer screening in women in Isfahan province, Iran (Persian)]. Journal of Health System Research. 2018; 13(4):415-21. [DOI:10.22122/jhsr.v13i4.2732]
- Saei Ghare Naz M, Mohaddesi H, Abed M, Darooneh T, Salmani F, Rashidi-Fakari F, Ghasemi V, et al. [Breast cancer screening practice and its relationship with breast cancer screening belief in women referred to health centers affiliated to Shahid Beheshti University of Medical Sciences, Tehran, Iran (Persian)]. Journal of Isfahan Medical School. 2018; 36(500):1234-41.[DOI: 10.22122/jims.v36i500.10790]
- Lam M, Kwok C, Lee MJ. Prevalence and sociodemographic correlates of routine breast cancer screening practices among migrant‐Australian women. Australian and New Zealand Journal of Public Health. 2018; 42(1):98-103. [DOI:10.1111/1753-6405.12752] [PMID]
- Irani M, Nosrati SF, Sardasht FG, Fasanghari M, Najmabadi KM. Knowledge, attitude, and practice of women regarding breast cancer screening behaviors in Mashhad, Iran. Journal of Midwifery & Reproductive Health. 2021; 9(2):2715. [Link]
- Nowak PF, Rogowska AM, Kwaśnicka A. The mediating role of health behaviors in the relationship between internal locus of control and life satisfaction in public health students. Scientific Reports. 2024; 14(1):19112. [DOI:10.1038/s41598-024-70178-z] [PMID]
- Saei Ghare Naz M, Darooneh T, Rashidi Fakari F, Kholosi Badr F, Hajizadeh F, Ozgoli G. The relationship between health locus of control and Iranian women’s beliefs toward pap smear screening. International Journal of Community Based Nursing and Midwifery. 2019; 7(1):43-51. [PMID]
- Hazavehei SMM, Ezzati-Rastegar K, Dogonchi M, Salimi N, Gheisvandi E. The impact of educational intervention programs to promoting mammography screening: A systematic review. Journal of Education and Community Health. 2016; 3(1):58-67. [DOI:10.21859/jech-03018]
- Saeed S, Asim M, Sohail MM. Fears and barriers: Problems in breast cancer diagnosis and treatment in Pakistan. BMC Women’s Health. 2021; 21(1):151. [DOI:10.1186/s12905-021-01293-6] [PMID]
- Khasareh H, Mirtajadini FS. [Prediction of Corona disease anxiety based on health locus of control and perceived social support (Persian)]. Rooyesh-e-Ravanshenasi Journal (RRJ). 2022; 10(12):97-108. [Link]
Type of Study: Orginal Article |
Subject:
● Disease Control
Received: 2024/10/31 | Accepted: 2025/03/5 | Published: 2025/11/1
Received: 2024/10/31 | Accepted: 2025/03/5 | Published: 2025/11/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |