Volume 15, Issue 6 And S7 (Artificial Intelligence 2025)
J Research Health 2025, 15(6 And S7): 779-792 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Barzegar M M, Ahmadi Daryakenari N, Khodatars M. Explainable Epileptic Seizure Detection from Electroencephalography Signals via CNN–Bi-LSTM Attention Hybrid Model. J Research Health 2025; 15 (6) :779-792
URL: http://jrh.gmu.ac.ir/article-1-2987-en.html
URL: http://jrh.gmu.ac.ir/article-1-2987-en.html
1- Department of Biomedical Engineering, School of Electrical Engineering, Iran University of Science and Technology (IUST), Tehran, Iran.
2- Department of Epileptology, University Hospital Bonn, University of Bonn, Bonn, Germany.
3- Department of Medical Engineering, MMS.C. Islamic Azad University, Mashhad, Iran. ,khodatars1marjane@gmail.com
2- Department of Epileptology, University Hospital Bonn, University of Bonn, Bonn, Germany.
3- Department of Medical Engineering, MMS.C. Islamic Azad University, Mashhad, Iran. ,
Full-Text [PDF 2404 kb]
(223 Downloads)
| Abstract (HTML) (1687 Views)
Full-Text: (34 Views)
Introduction
Epilepsy is a chronic neurological disorder characterized by frequent seizures due to abnormal electrical activity in the human brain [1]. The World Health Organization (WHO) has reported that more than 50 million people worldwide suffer from epileptic seizures [1]. According to this report, epileptic seizures are the third most common brain disorder, following stroke and Alzheimer’s disease (AD) [1, 2]. These seizures negatively affect the human nervous system and lead to various challenges in the daily lives of patients, such as movement disorders, lack of bladder control, and loss of consciousness [2, 3]. Such issues can be very dangerous for individuals with epilepsy, potentially resulting in paralysis, fractures, or even death [3]. Due to their unpredictability, epileptic seizures often lead to fear, anxiety, stress, and a decrease in patients’ self-confidence [3]. Specialists believe that diagnosing epileptic seizures in their early stages can help treat over 70% of affected individuals [4].
Electroencephalography (EEG) is one of the most well-known methods for diagnosing epileptic seizures among specialist doctors [2-4]. EEG records brain activity during epileptic seizures from the scalp non-invasively [1]. In addition, EEG recording is very popular among neurologists and researchers due to its low cost and easy portability compared to other neuroimaging modalities [3]. EEG measures electrical currents in the dendrites of neurons that are close to the surface of the cerebral cortex with high resolution [4]. Currently, specialist doctors visually extract information from EEG signals to diagnose epileptic seizures. In this process, neurologists can diagnose the condition based on important information in EEG signals, including spikes, sharp waves, and slow waves [5]. Therefore, this method is highly dependent on the experience of doctors specializing in the analysis of EEG signals [5].
Visual analysis of EEG signals is always challenging for neurologists due to the variety of epileptic seizures. Additionally, EEG signals are usually recorded under different conditions, such as with EEG devices that have different sampling frequencies, along with various artifacts from patients. This variability makes it difficult to diagnose epileptic seizures accurately. Misdiagnosis of epileptic seizures by specialist doctors can cause irreparable damage to patients [4, 5]. For example, epileptic seizures are generally classified into two categories: focal and generalized [6]. Misdiagnosis of the type of epileptic seizure can lead to the prescription of inappropriate medications, which may result in drug-resistant epilepsy and, ultimately, death [4, 5]. Therefore, diagnosing epileptic seizures at their early stages from EEG signals is vital for specialist doctors. Over the years, the use of artificial intelligence (AI) techniques, especially machine learning (ML) methods [7, 8] and deep learning (DL) networks [9, 10], to assist in detecting epileptic seizures from EEG signals has grown significantly.
In recent years, extensive research has been conducted in the field of diagnosing epileptic seizures through computer-aided diagnosis systems (CADS) [7-10]. An AI-based CADS consists of a dataset, preprocessing, feature extraction and selection, as well as classification [9, 10]. Feature extraction is the most crucial part of a CADS for detecting epileptic seizures from EEG signals. Until 2016, most researchers focused on CADS utilizing ML techniques [7, 8]. In the field of ML, researchers have presented various methods to extract features from EEG signals aimed at improving the accuracy of diagnosing epileptic seizures. ML feature extraction techniques include time domain, frequency domain, time-frequency domain, and nonlinear transformation methods [3, 4]. In ML-based CADS, researchers often combine features from different domains to enhance the accuracy of epileptic seizure diagnosis. This work is typically performed through trial and error and is highly dependent on the individual’s expertise in the field of ML [3, 4].
With the advent of DL techniques, these networks have quickly replaced ML methods in various medical applications, particularly in the diagnosis of epileptic seizures [9, 10]. Compared to ML techniques, DL networks have achieved promising results in detecting epileptic seizures from EEG data. While DL models offer numerous advantages, they also have some disadvantages, including high computational costs and the need for expensive GPU processors [11]. Nevertheless, researchers are eager to implement DL architectures in the diagnosis of epileptic seizures due to their significant features, such as the ability to automatically extract features from EEG signals [10]. The most important DL architectures used in applications for epileptic seizure detection include convolutional neural networks (CNNs) [12], recurrent neural networks (RNNs) [13], autoencoders (AEs) [14], deep belief networks (DBNs) [15], attention mechanisms [16], and graph models [17]. The development of DL architectures provides hope for researchers, as they aim to implement practical tools for diagnosing epileptic seizures from EEG signals in hospital environments and specialized clinics in the future.
Attention mechanism architectures are a new category of DL techniques that have achieved significant results in the diagnosis of brain disorders [18, 19]. Unlike other DL networks, these networks perform well with limited input data. These architectures focus on specific regions of EEG signals that contain important information, allowing for the extraction of the most critical features from EEG signals. Transformer models represent a new class of attention mechanism networks and are widely used as powerful tools in the analysis of time series, such as EEG signals [20, 21]. In this paper, we presented a new method for diagnosing epileptic seizures based on 1D-CNN Bi-LSTM attention mechanism. First, the EEG signals underwent several preprocessing steps, including filtering, down-sampling, re-referencing using common average reference (CAR), and segmenting the data. Subsequently, the proposed DL architecture was employed to extract features from the preprocessed EEG signals. After that, Sigmoid was utilized to classify the input data, and their results were compared. Finally, the t-distributed stochastic neighbor embedding (t-SNE) technique and shapley additive explanations (SHAP) [25, 26] were used as an explainable artificial intelligence (XAI) technique to represent the feature space.
Related works
In recent years, numerous studies have been conducted in the field of diagnosing epileptic seizures from EEG signals using AI techniques, particularly DL networks. In all these articles, the main goal of the researchers has been to find new DL methods to assist in the early diagnosis of epileptic seizures. Below, several articles that focus on diagnosing epileptic seizures using novel DL models are examined.
Samee et al. utilized a combination of RNN and bidirectional long short-term memory (Bi-LSTM) architectures to detect epileptic seizures [27]. In their work, the Bonn dataset was selected for the simulations. The authors employed EEG signal windowing in the preprocessing step. They then proposed a DL model that fuses RNN with Bi-LSTM to extract features from EEG signals. Finally, they used the Softmax function to classify the extracted features and achieved acceptable results.
In another study, Choi et al. proposed a new DL architecture based on an attention mechanism for diagnosing epileptic seizures [28]. First, EEG signals were preprocessed through filtering, segmentation, and normalization. Subsequently, 1D convolutional neural networks (1D-CNN), gated recurrent units (GRU), and attention mechanism networks were employed for feature extraction and classification. Their proposed DL architecture aimed to extract spatial and temporal features to enhance the accuracy of epileptic seizure diagnosis from EEG signals. Finally, the Softmax activation function was utilized in the last fully connected (FC) layer of the proposed DL architecture for classification.
A novel method for detecting epileptic seizures from EEG signals using a graph convolutional neural network (GCNN) model was introduced by Jia et al. [29]. The preprocessing steps in their work included both low- and high-level processes. Low-level preprocessing involved filtering, normalization, and segmentation. Following this, various features were extracted from EEG signals as part of the high-level preprocessing step. Finally, a GCNN model with the Softmax function was employed for feature extraction and classification, respectively.
In another study, Wang et al. proposed a new DL architecture to detect epileptic seizures [30]. This architecture consists of two dynamic multi-graph convolution networks (DMGCN) and a channel-weighted transformer (CWTr) to extract features from EEG signals. In their research, the CHB-MIT dataset was chosen for the experiments, during which the EEG signals were decomposed into different time windows in the preprocessing step. The proposed DL architecture was then applied in the feature extraction stage. Finally, the Softmax function was used in the classification step, enabling the researchers to achieve satisfactory results.
In another research [31], the authors proposed a method for detecting epileptic seizures from EEG signals using a transformer architecture. In the preprocessing step, normalization and windowing were first applied to the EEG signals of the CHB-MIT dataset. Then, the EEG signals were converted into 2D images using the short-time fourier transform (STFT) method. Next, feature extraction from the 2D images was performed using a hybrid transformer model. Finally, in the classification step, the Softmax algorithm was employed in the last FC layer of the proposed DL architecture.
Jibon et al. introduced a graph deep learning (GDL) architecture to extract features from EEG signals [32]. In this work, simulations were conducted on the TUH and CHB-MIT datasets. The preprocessing of EEG signals includes both low-level and high-level components. Low-level preprocessing involves filtering and windowing, while high-level preprocessing includes EEG signal decomposition, feature extraction, and graph representation. Subsequently, sequential graph convolutional network (SGCN) and RNN architectures were utilized for the feature extraction and classification stages.
In another study, researchers presented a method for diagnosing epileptic seizures using dynamic brain functional connectivity [33]. In this work, the TUH dataset was first selected for experiments. Next, EEG signals were preprocessed using a functional connectivity method to convert the EEG signals into 2D images. They then employed a graph-generative neural network (GGNN) architecture to extract features from the 2D functional connectivity images. Finally, the Softmax function was applied to classify the input features.
A new method for detecting epileptic seizures based on a linear graph convolutional network (LGCN) and DenseNet was presented in another study [17]. In the preprocessing step, the authors separated the EEG signals into different time intervals. Following this, they applied the Stockwell transform and graph structure methods to the EEG window signals. Next, LGCN and DenseNet architectures were integrated and employed for feature extraction and classification. The results of this work demonstrated that the authors achieved significant success in diagnosing epileptic seizures.
Ma et al. [34] extracted temporal and spatial features using a proposed DL architecture for the detection of epileptic seizures. In this study, the CHB-MIT and UCI datasets were selected, after which preprocessing steps, including normalization and one-hot encoding, were applied. The proposed DL architecture consists of 1D-CNN and Bi-LSTM blocks with an attention mechanism to extract features from EEG signals. This part is capable of extracting both spatial and temporal features simultaneously. In this work, the authors achieved acceptable accuracy in diagnosing epileptic seizures.
In another study, Lih et al. [35] employed a transformer architecture with the aim of improving the diagnosis of epileptic seizures. In this research, they presented a new dataset with 121 subjects, including two classes: seizure and healthy control (HC). In the preprocessing step, the EEG signals were first segmented into different time windows, after which the Pearson correlation coefficient (PCC) was calculated for each EEG segment. Next, feature extraction and classification steps were performed using a proposed transformer architecture on the 2D PCC images. The most significant innovation of this research lies in the data section.
Methods
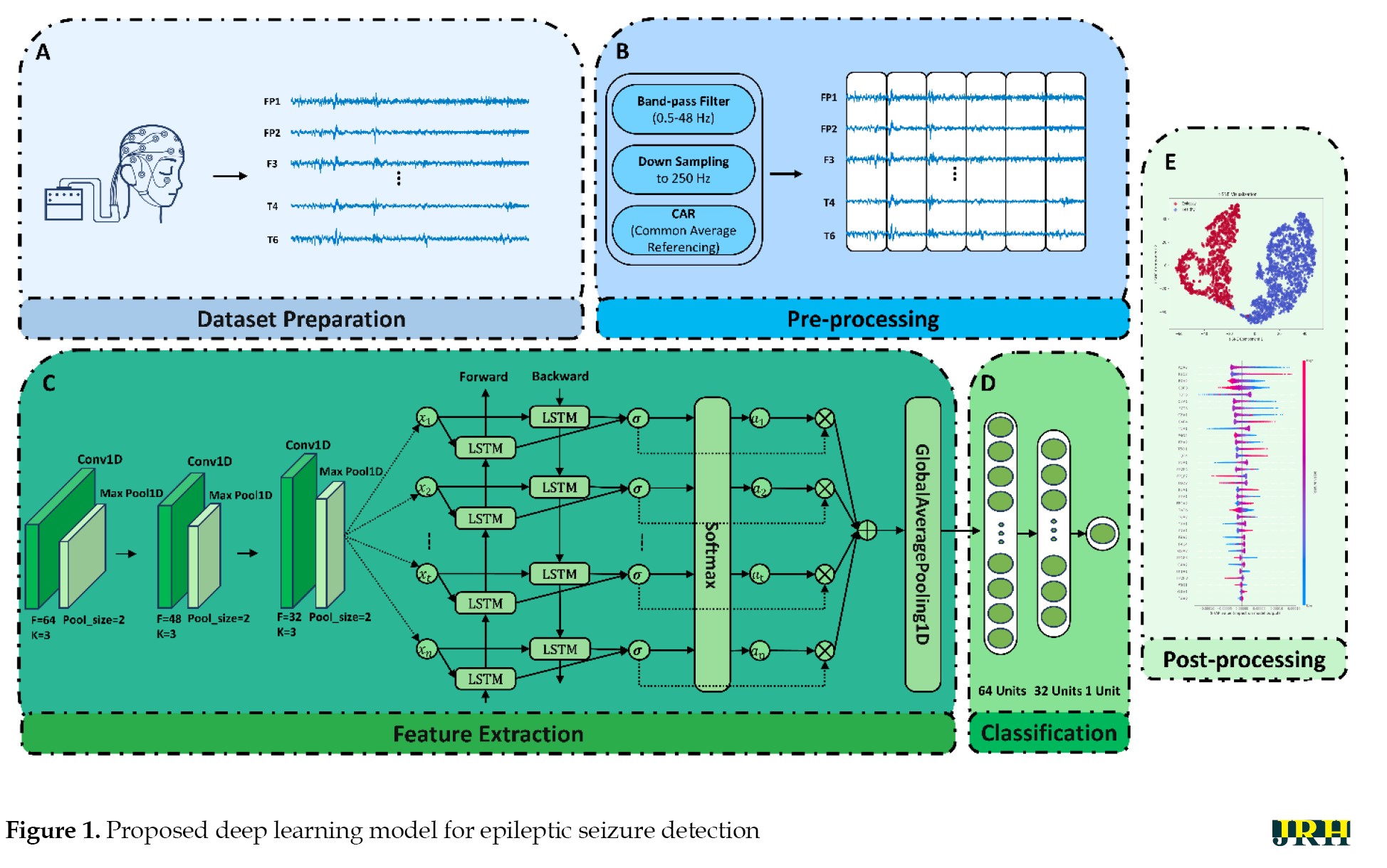
In this section, the proposed method for diagnosing epileptic seizures based on EEG signals is presented. In Figure 1, details of the proposed CADS sections based on a new DL network are shown. As illustrated, the proposed method consisted of dataset preparation, preprocessing, feature extraction, classification, and post-processing sections. The simulation of the proposed method was performed using the Turkish epilepsy EEG dataset, which included 121 subjects divided into two classes: epileptic seizures and HC [35]. Preprocessing steps, including filtering, down-sampling, re-referencing using CAR, and segmentation, were applied to the EEG signals. In the next step, a proposed DL architecture, which consists of a 1D-CNN Bi-LSTM fused with an attention mechanism, was employed to extract spatio-temporal features from the preprocessed EEG signals. EEG signals are non-linear and spatio-temporal in nature [36-38]. The 1D-CNN architecture extracts spatial features from the EEG signals, while the Bi-LSTM network captures temporal dependencies. The attention mechanism helps the model focus on the most relevant parts of the signal, thereby increasing the accuracy of diagnosing epileptic seizures from EEG signals. Finally, t-SNE and SHAP were utilized as an XAI technique in the post-processing step to visualize the feature space extracted by the proposed DL architecture [25, 26].
Dataset and preprocessing
As mentioned, the Turkish epilepsy dataset was selected to perform simulations and assess the performance of the proposed DL architecture. In this dataset, EEG data were recorded from 121 subjects, of whom 50 experienced epileptic seizures while 71 were classified as HC [35]. EEG signals were recorded from the parietal, frontal, temporal, occipital, frontopolar, auricular, and central regions. Data recording was conducted using the 10-20 electrode placement standard, with 35 channels and a sampling frequency of 500 Hz [35]. In this section, first, a band-pass filter (0.5–48 Hz) was applied to eliminate artifacts and retain frequency components of interest. Further, the EEG signals were down-sampled to 250 Hz. CAR was then used to enhance the signal-to-noise ratio by subtracting the mean activities across all channels from each individual channel; this would suppress common noises. Finally, the preprocessed EEG signals were divided into non-overlapping windows of 5 s, 10 s, and 15 s duration.
DL model
Today, DL architectures are increasingly being used in the field of diagnosing epileptic seizures from EEG signals. 1D-CNNs are an important category of DL models that effectively extract spatial features from EEG signals; however, they overlook the temporal correlations between EEG channels [36, 37]. RNNs represent another category of DL frameworks that are widely used for feature extraction from EEG signals. RNN models are generally more successful at capturing temporal features compared to CNN models [21]. Researchers have demonstrated that CNN-RNN architectures can successfully extract both spatial and temporal features from EEG signals. However, these architectures lack a mechanism to identify the importance of specific parts of the input EEG signals, such as critical channels, which could improve the accuracy of epileptic seizure detection. Consequently, CNN-RNN architectures frequently face challenges in the simultaneous extraction of spatio-temporal features. To address this issue, attention mechanism architectures have been introduced to overcome the limitations of RNN models in feature extraction from EEG signals. In this paper, we proposed an improved architecture based on the 1D-CNN Bi-LSTM Attention to extract both spatial and temporal features from EEG signals.
1D-CNN module
The presented model initially used 1D-CNN blocks, which are responsible for extracting spatial features from EEG raw signals. The basic operations inside these convolutional blocks were formally described as follows (Equation 1):
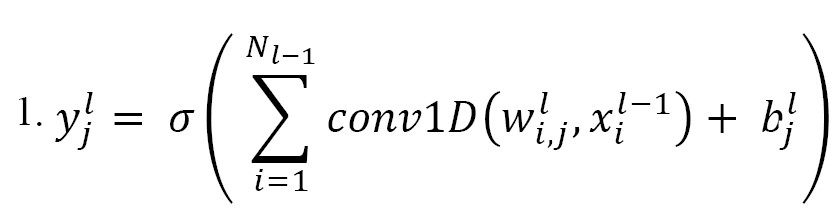
where denotes the i-th feature map in the (l − 1)-th layer; denotes the j-th feature map in the l-th layer, while represents the trainable convolutional kernel; represents the number of feature maps in the (l-1)-th layer; conv1D represents the 1D convolution operation without zero-padding; represents the bias of the j-th feature map in the l-th layer; and denotes the ReLU activation function, which is used to suppress overfitting and increase the non-linearity of the model. It is defined as follows (Equation 2):
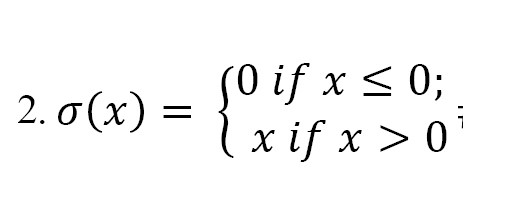
The max-pooling operation in layer was defined as follows (Equation 3):
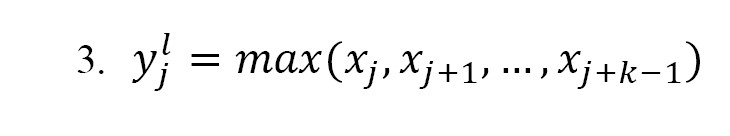
In this operation, a sliding window of size k was applied over the input sequence. For each position j, the max-pooling function selects the maximum value within the window. Max-pooling contained no trainable parameters and its purpose was to reduce the dimensionality of the feature representation.
Table 1 summarizes the specifications of the 1D-CNN Bi-LSTM Attention architecture, which comprises a total of 17 layers.
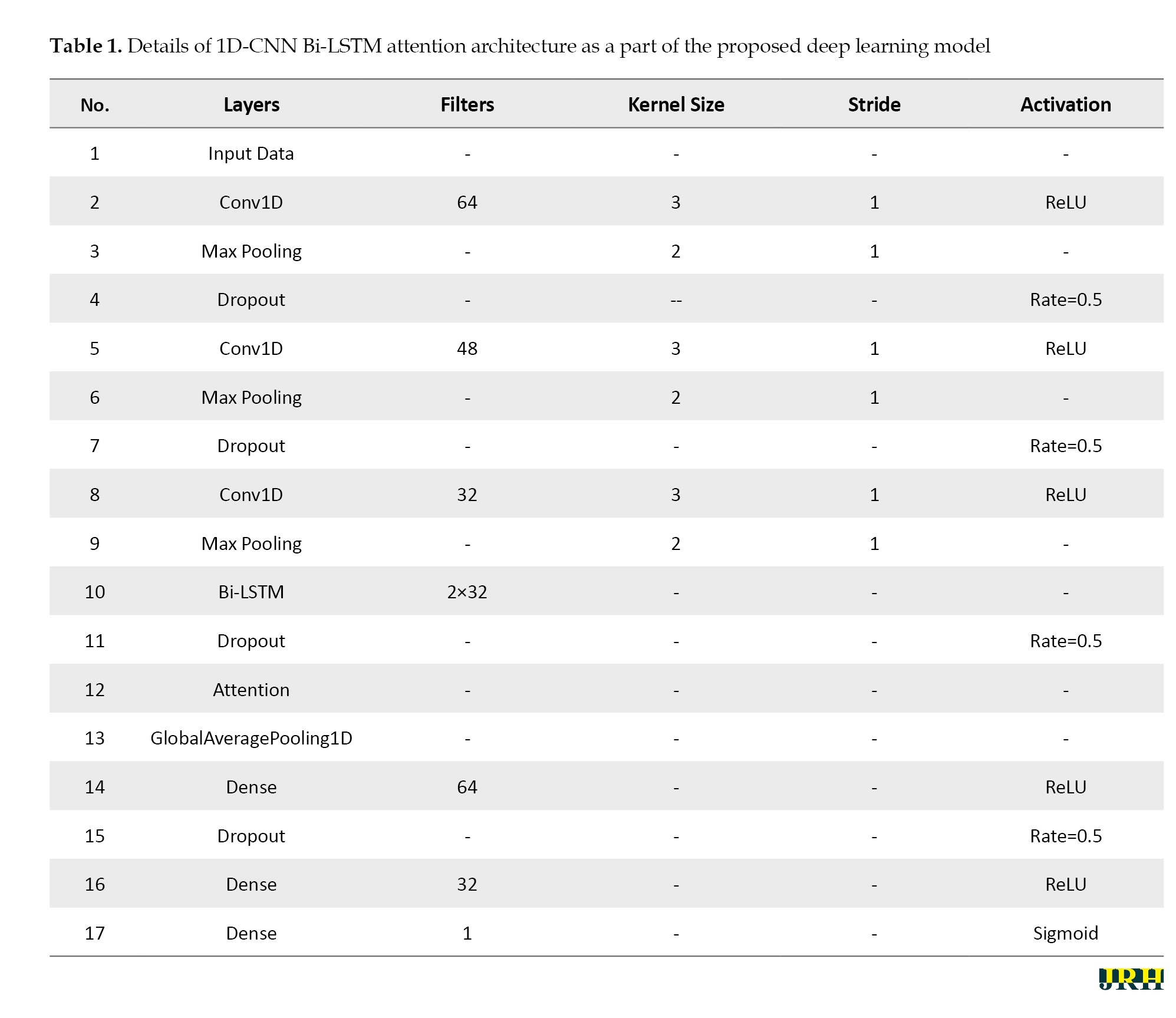
The input data first passes through a 1D convolutional layer with 64 filters, a kernel size of 3, a stride of 1, and ReLU for activation. This was followed by a max-pooling layer to reduce the dimensionality by half, and a dropout layer with a rate of 0.5 was used to control overfitting. Next, another 1D convolutional layer with 48 filters, a kernel size of 3, a stride of 1, and ReLU for activation was used. Similar to the previous block, this layer is followed by a max-pooling layer and a dropout layer with a rate of 0.5. A third convolutional layer, containing 32 filters, a kernel size of 3, a stride of 1, and ReLU for activation, is then used; this is again followed by a max-pooling layer with a pool size of 2. These convolutional, pooling, and dropout operations extracted hierarchical spatial features in the EEG signals while controlling overfitting. The output of the last block was fed into the next subsequent modules of the network for processing.
Bi-LSTM module
After the 1D-CNN feature extraction, the output feature sequences were utilized by a Bi-LSTM network, which is able to identify the temporal dynamics of the EEG signals. The main operation of the Bi-LSTM network is based on the functions performed inside the LSTM unit. The activities that happen in the main LSTM unit at time are mathematically described below (Equation 4):
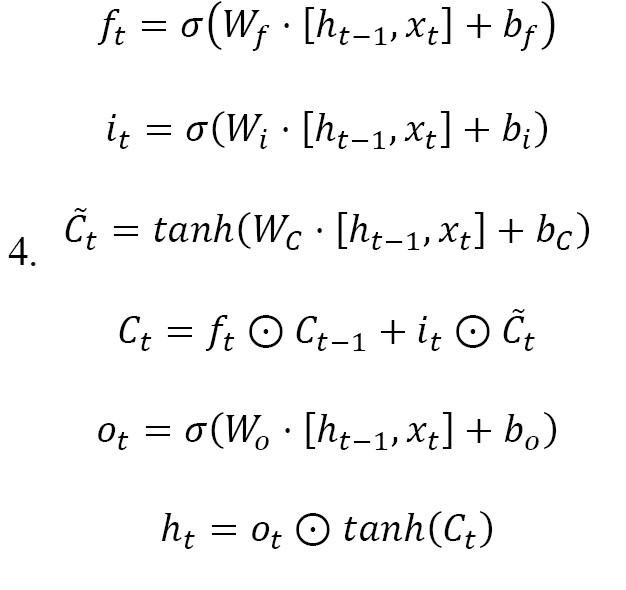
Where xt symbolizes the input feature vector, ht-1 indicates the prior hidden state, ct-1 represents the prior cell state, σ is the Sigmoid function, and is the Hadamard product. The Bi-LSTM architecture processes the input in both forward ht and backward ht directions, enabling the model to effectively learn long-range dependencies in the temporal domain. Each time step is passed through two parallel LSTM units—one moving from past to future and the other from future to past—providing a richer representation of temporal patterns. The Bi-LSTM has 32 hidden units in both directions. The final output vector Ht of the Bi-LSTM layer is the concatenation of the forward and backward hidden states (Equation 5):
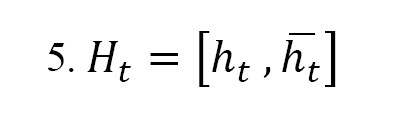
Attention mechanism module
The Bi-LSTM layer generates activation vectors that will be input into an attention mechanism built to help accentuate the most relevant temporal variables. The mathematical formulation of the attention mechanism is defined as follows, where is the output matrix of the Bi-LSTM (Equation 6):
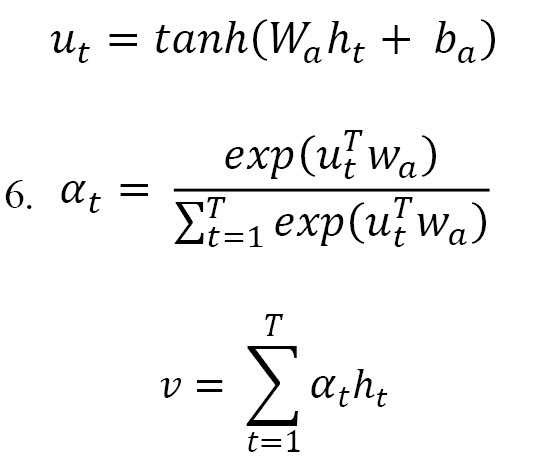
The attention layer produced a distribution of attention weights that favor the time steps most applicable to the classification task. This was accomplished by computing all of the Bi-LSTM outputs ht and multiplying them by their associated attention weight, allowing the Bi-LSTM to dynamically adjust the focus of learning on the relevant temporal components of the data and filter out any noise or otherwise unhelpful data. A Global Average Pooling 1D layer then aggregated these weighted representations into a compact feature vector, which was subsequently forwarded to the FC layers for final classification.
Experiments
Hardware and software resources
In this section, we reported the results of the proposed DL architecture for epileptic seizure detection from EEG signals. All simulations in this study were conducted using a hardware system equipped with an NVIDIA 1070 GPU, 512 GB of RAM, and a Core i7 CPU. Additionally, TensorFlow [40] and Scikit-learn tools [40] were used for implementing the DL architecture and calculating the evaluation metrics, respectively. The implementation of the t-SNE technique [25] was also carried out using the Scikit-learn library [40].
Evaluation metrics
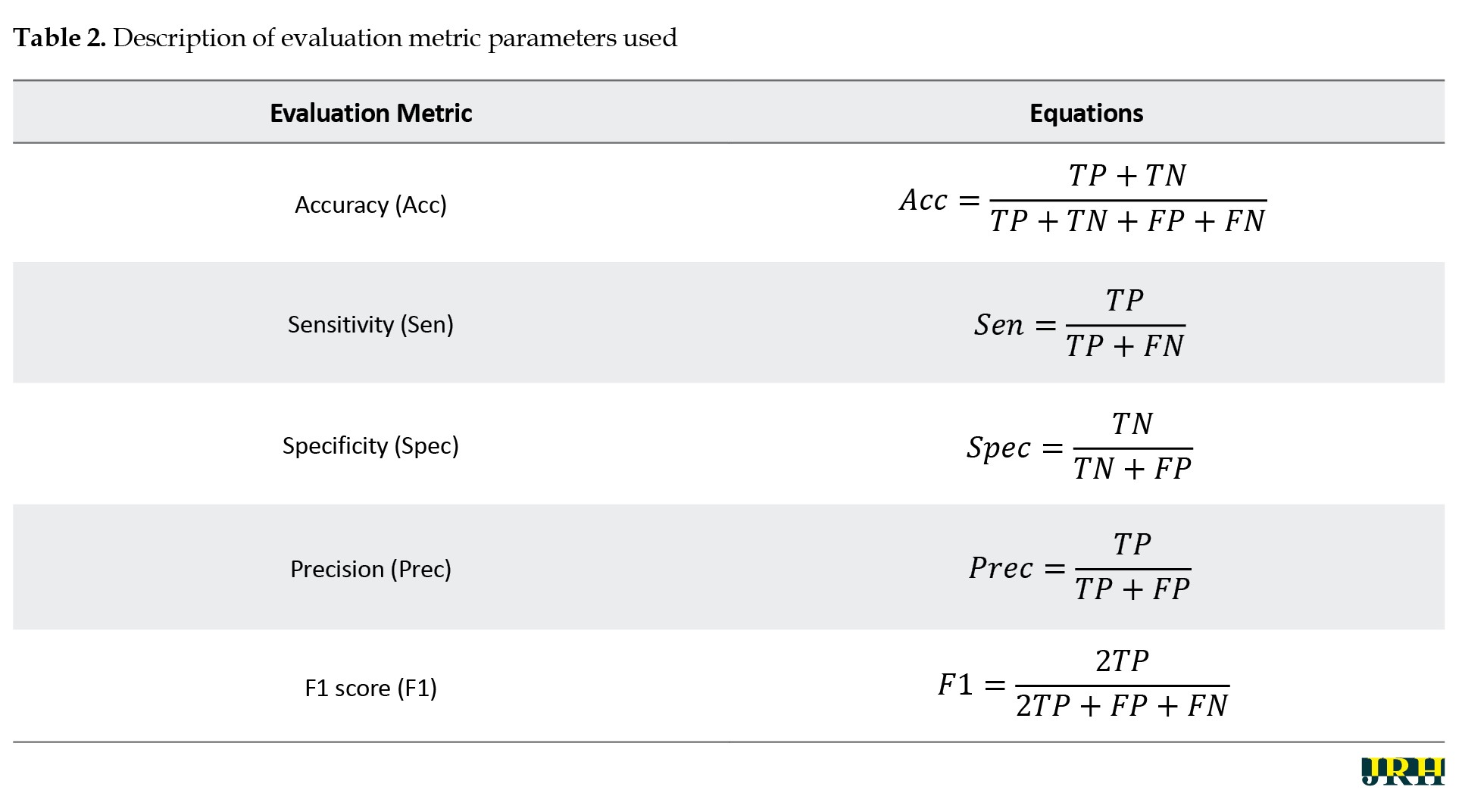
Calculating evaluation metrics is essential for evaluating the performance of the proposed DL architecture for epileptic seizure diagnosis. In this section, evaluation measures, including accuracy (Acc), sensitivity (Sen), specificity (Spec), precision (Prec), and F1 score (F1) were calculated. The formulas for these evaluation metrics are briefly reported in Table 2.
To implement the proposed DL model, 5-fold cross-validation was applied to the entire EEG dataset. In each fold, 80% of the data was used for training and the remaining 20% served as the test set. After that, the aforementioned evaluation metrics were calculated for the training, testing, and validation data. For this purpose, the Scikit-learn library [40] was employed to compute the evaluation metrics in a Python environment. Given the importance of data validation, this study reported the results of validation data in the experimental results section.
Implementation details
The hyperparameters of the DL network proposed to achieve optimal performance are presented below. The number of epochs, batch size, and regularization (L1) were set at 100, 32, and 0.0009, respectively. Furthermore, the Adam optimization method and binary cross-entropy were utilized as the cost function in the implementation of the proposed DL network. Additionally, the Sigmoid activation function was employed as the classification step.
Results
This section presents the results of the proposed DL architecture for detecting epileptic seizures from EEG signals. Tables 3, 4 and 5 show the performance of different DL architectures applied to EEG signals with various time windows. For each EEG time frame, the results of the 1D-CNN, 1D-CNN Bi-LSTM, and 1D-CNN Bi-LSTM attention models were compared.
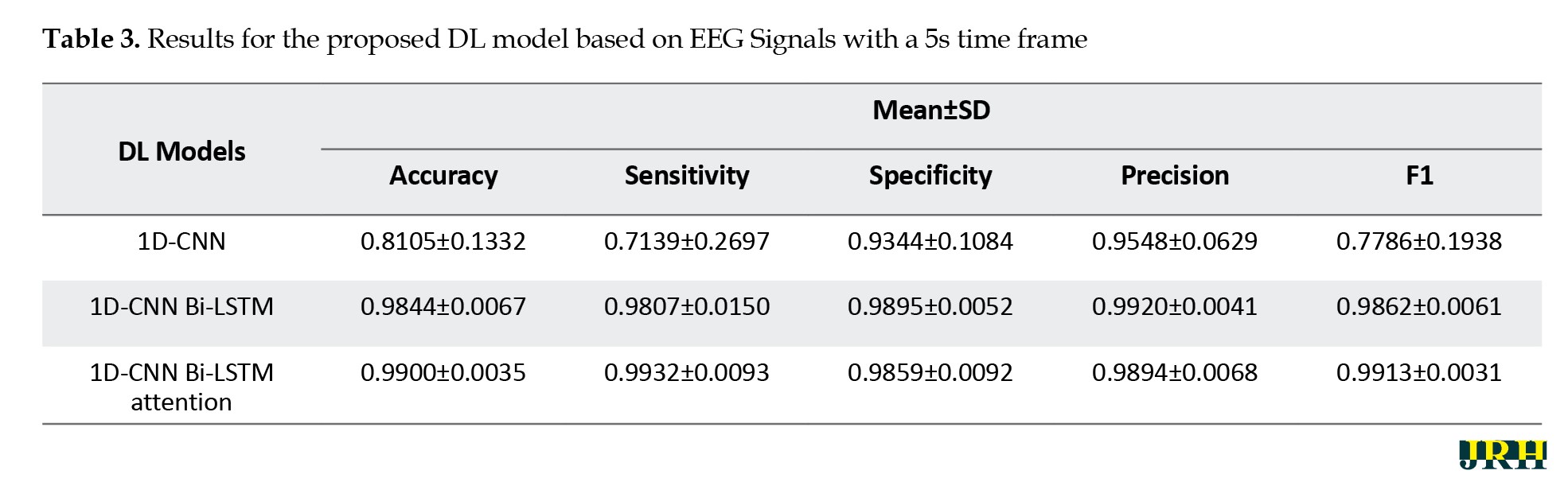
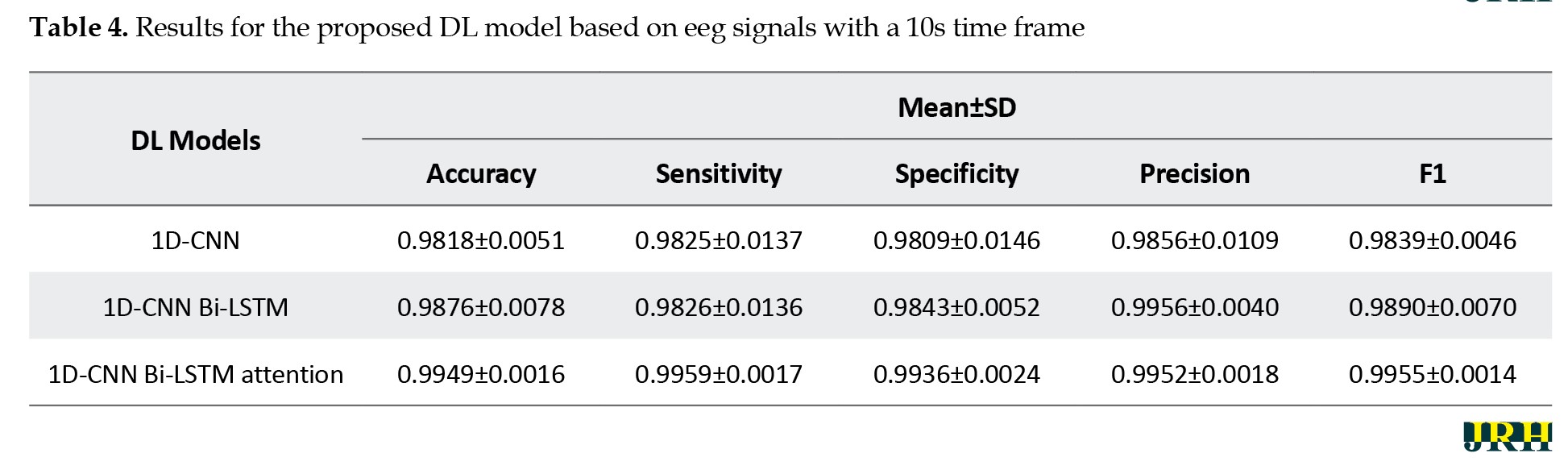
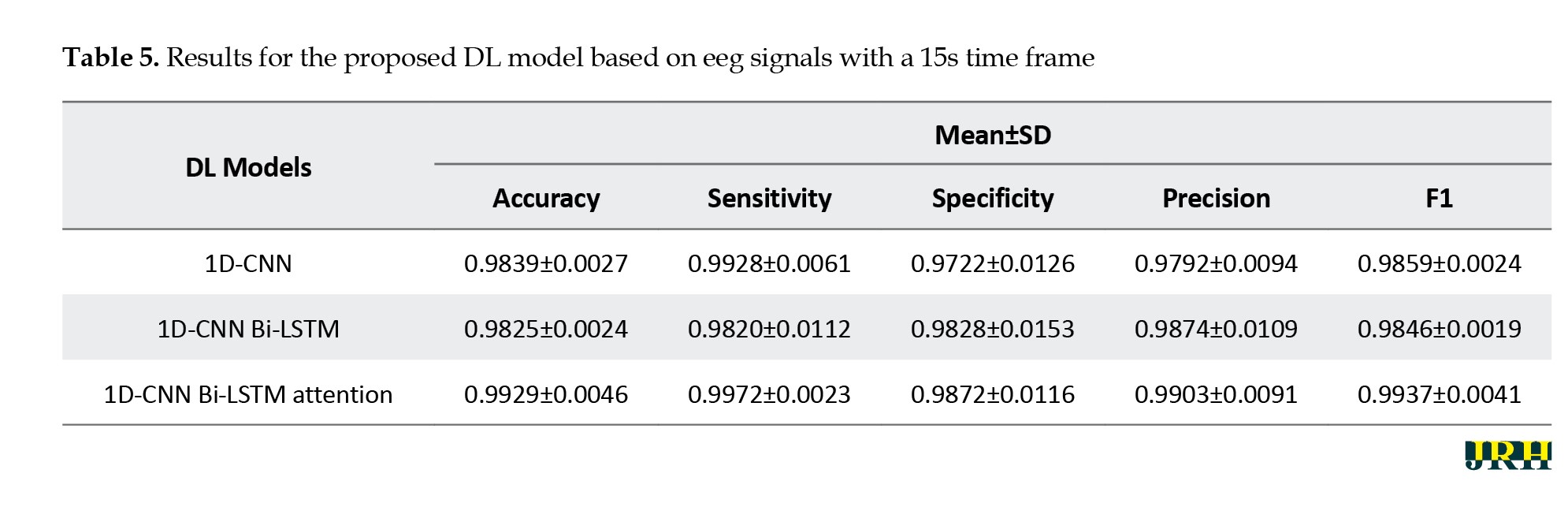
According to Tables 3, 4 and 5, the proposed 1D-CNN Bi-LSTM attention architecture with a 10-second frame achieved the highest accuracy in detecting epileptic seizures.
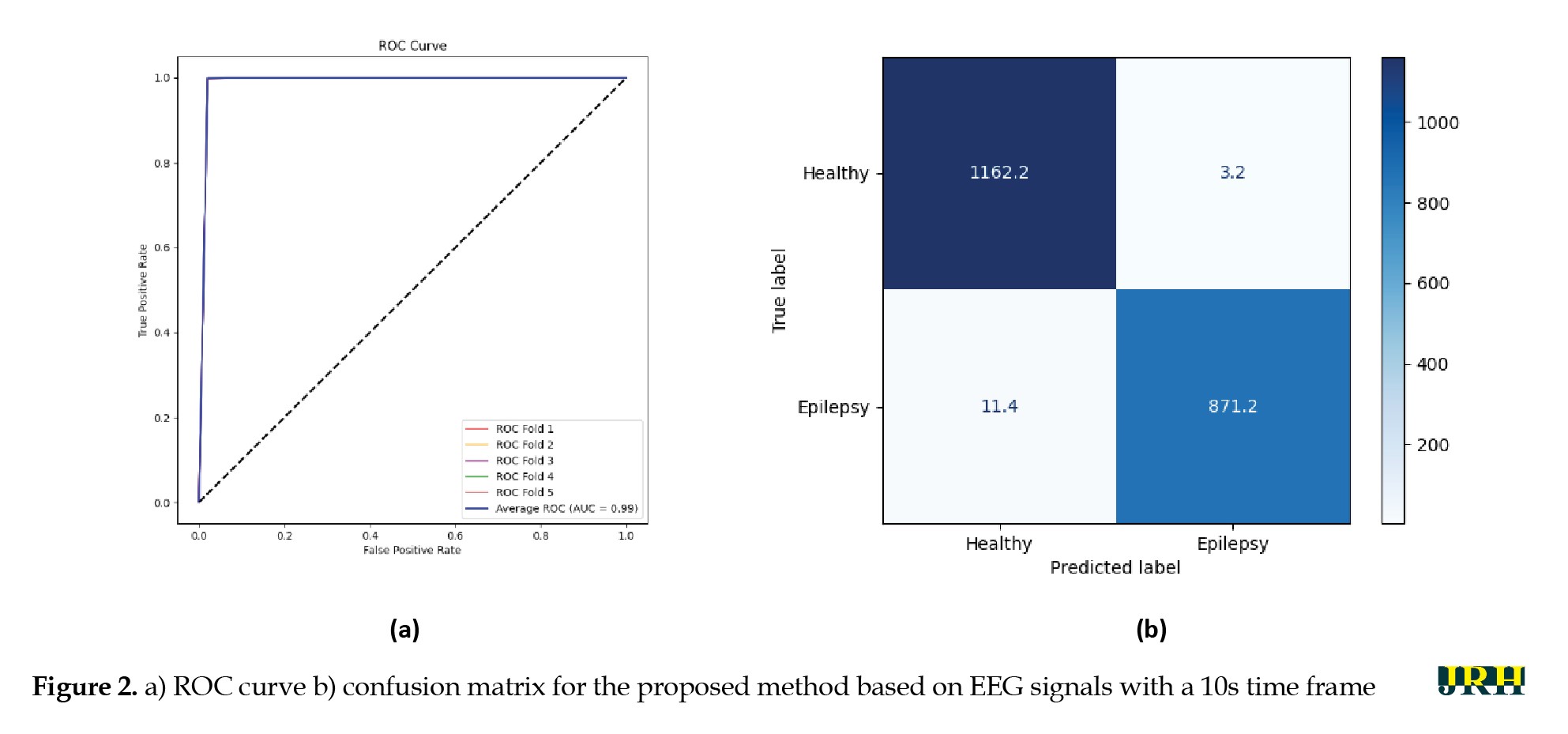
Accordingly, additional results for the proposed DL architecture with a 10-second frame are presented below. Figure 2 illustrates the ROC curve and confusion matrix of the proposed 1D-CNN Bi-LSTM attention model based on EEG signals with a 10-second time frame. As shown, the proposed DL architecture achieved strong performance in epileptic seizure detection.
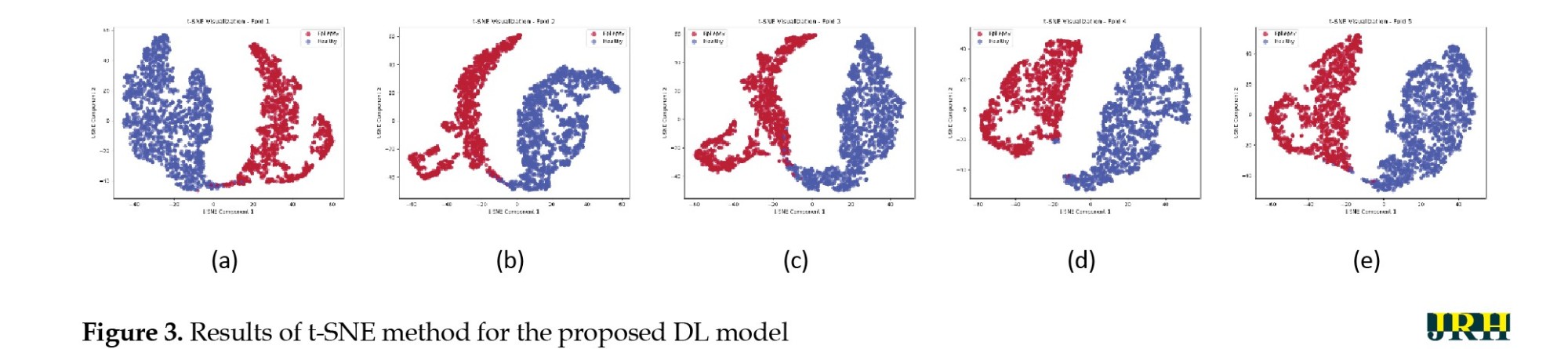
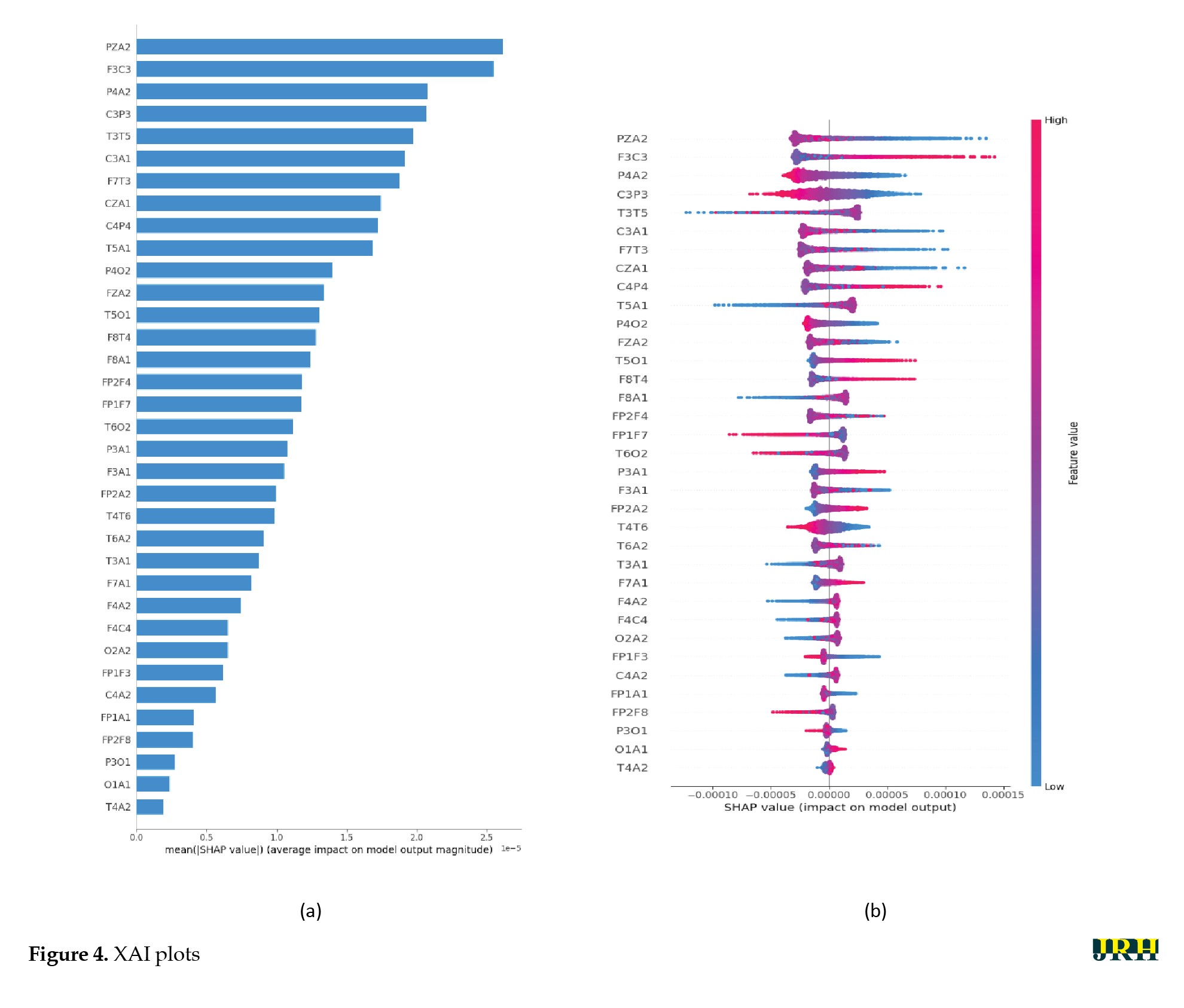
Next, the results of XAI methods, including t-SNE and SHAP models, are presented to provide further insights into the proposed method [25, 26]. By reducing high-dimensional EEG data to a lower-dimensional space, t-SNE facilitates the visualization of complex patterns and relationships in seizure data, enabling clinicians to understand the fundamental features that distinguish different types of seizures. This interpretability is critical for practitioners, as it provides insights into the model’s decision-making process and allows for a more informed evaluation of the classifications produced by the proposed DL model. Figure 3 displays the results of the t-SNE method for the proposed DL architecture based on 5-fold cross-validation technique. The interpretability of the proposed model was assessed using SHAP, which is a unified framework to explain predictions based on cooperative game theory. SHAP assigns a unique importance value to each feature, representing its average marginal contribution to the model’s prediction across all possible feature combinations. This, in turn, helps us understand the local behavior of which specific features drive individual classification decision [26]. The results of the SHAP model as an XAI technique are shown in Figure 4. Figure 4a presents the SHAP summary bar plot, which displays the absolute mean SHAP values for each feature. This identifies the most influential EEG channels—PZA2, F3C3, P4A2, C3P3, and T3T5—in detecting epileptic seizures using the proposed DL model. These channels were located in the frontal, temporal, and parietal regions, which is consistent with neurophysiological evidence. Figure 4b also shows the SHAP Beeswarm plot, which illustrates the full distribution of SHAP values for each feature and indicates how increases or decreases in feature values affect the probability of seizure detection. The color of the dots represents feature values (blue for low, red for high). For important features, such as PZA2, F3C3, and P4A2, high feature values (red points) generally correspond to positive SHAP values, thereby increasing the likelihood of seizure prediction. This behavior aligns with typical EEG patterns during seizures, including increased amplitude, sharp waves, and burst activity.
Discussion
Epileptic seizures are among the most well-known neurological disorders caused by abnormal electrical discharges in brain neurons [2-4]. This condition is associated with transient seizures throughout the day and poses serious health risks to patients, including fainting, anesthesia, and loss of muscle control [1-3]. Generally, neurologists diagnose epileptic seizures by examining abnormal amplitudes on EEG waveforms, a task that is very time-consuming and associated with human error [7, 8]. Because EEG signals are non-linear and contain various artifacts, their visual analysis is challenging for expert clinicians. Additionally, EEG signals are usually recorded over long periods and with different sampling frequencies, making accurate visual observation extremely time-consuming for doctors [9-11]. Moreover, EEG signals are captured through multiple channels, further complicating the data used for diagnosing epileptic seizures.
In recent years, AI techniques, particularly DL models, have garnered attention from researchers for diagnosing epileptic seizures from EEG signals [9, 10]. This study introduced a new method for diagnosing epileptic seizures based on a 1D-CNN Bi-LSTM attention architecture. The proposed method encompassed several sections: dataset preparation, preprocessing, feature extraction, classification, and post-processing. The implementation and evaluation of this method were conducted using a Turkish epilepsy dataset [35]. Initially, preprocessing steps, such as band-pass filtering (0.5–48 Hz), down-sampling to 250 Hz, re-referencing using CAR, and segmenting were applied to the EEG signals. Subsequently, the proposed DL model was implemented for extracting spatial-temporal features from the preprocessed EEG signals. Finally, the t-SNE method and SHAP [25, 26] were employed as a post-processing step to visualize the space of the extracted features. In Table 6, we presented the results of papers on diagnosis of epileptic seizures and compared them with our proposed method.
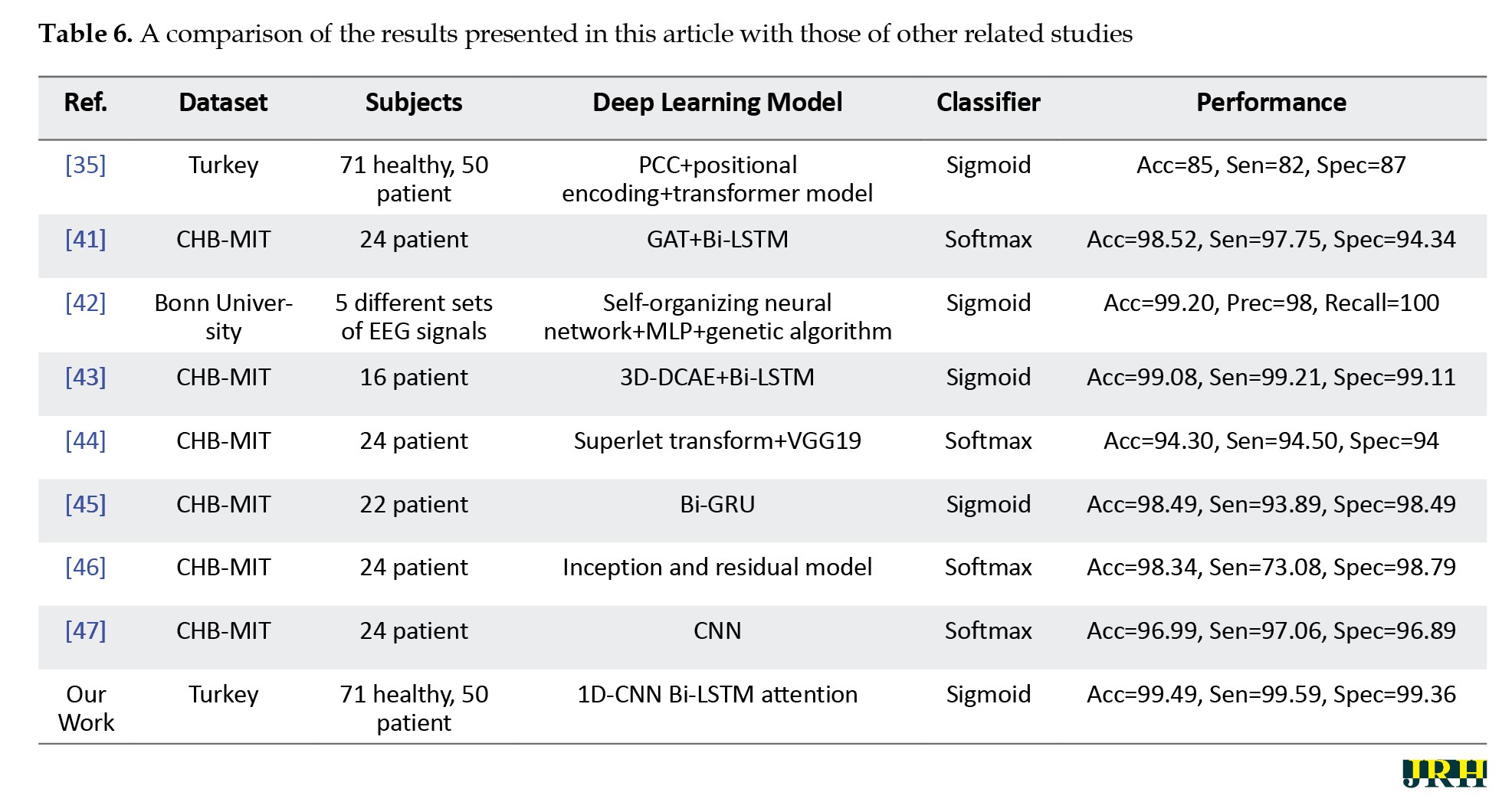
As observed, our proposed method demonstrated significant results compared to other research.
In the future, the proposed method could serve as practical software in hospital settings to assist specialist doctors in the rapid diagnosis of epileptic seizures based on EEG signals. Recently, some researchers have utilized new attention mechanism architectures, especially attention-graph models [48, 49] and transformer models with mutual learning architectures [50, 51], in medical applications. For future work, these DL networks could be applied to the diagnosis of epileptic seizures. It has been demonstrated that Graph DL networks have been highly successful in diagnosing brain disorders from EEG signals [52, 53]. For further work, the use of new graph architectures, such as multi-layer graph attention networks (MGANet) [54], spatial-temporal graph attention networks with transformer encoders (STGATE) [55], and adaptive gated graph convolutional networks (AGGCN) [56] should be considered for detecting epileptic seizures.
Conclusion
In conclusion, epileptic seizures pose significant health challenges and are traditionally diagnosed through time-consuming and error-prone EEG analysis. The integration of advanced deep learning models, such as the proposed 1D-CNN Bi-LSTM attention architecture, demonstrates promising improvements in accurately detecting seizures from EEG signals. The study’s results indicate that this approach outperforms existing methods, highlighting its potential for clinical application. Future research should explore incorporating cutting-edge attention mechanisms and graph-based neural networks to further enhance diagnostic accuracy and facilitate practical deployment in medical settings.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Data extraction and Simulation: Mohammad Mehdi Barzegar; Review and editing: Mohammad Mehdi Barzegar and Marjane Khodatars; Conceptualization, supervision, and writing the original draft: All authors.
Conflict of interest
The authors declared no conflicts of interest.
References
Epilepsy is a chronic neurological disorder characterized by frequent seizures due to abnormal electrical activity in the human brain [1]. The World Health Organization (WHO) has reported that more than 50 million people worldwide suffer from epileptic seizures [1]. According to this report, epileptic seizures are the third most common brain disorder, following stroke and Alzheimer’s disease (AD) [1, 2]. These seizures negatively affect the human nervous system and lead to various challenges in the daily lives of patients, such as movement disorders, lack of bladder control, and loss of consciousness [2, 3]. Such issues can be very dangerous for individuals with epilepsy, potentially resulting in paralysis, fractures, or even death [3]. Due to their unpredictability, epileptic seizures often lead to fear, anxiety, stress, and a decrease in patients’ self-confidence [3]. Specialists believe that diagnosing epileptic seizures in their early stages can help treat over 70% of affected individuals [4].
Electroencephalography (EEG) is one of the most well-known methods for diagnosing epileptic seizures among specialist doctors [2-4]. EEG records brain activity during epileptic seizures from the scalp non-invasively [1]. In addition, EEG recording is very popular among neurologists and researchers due to its low cost and easy portability compared to other neuroimaging modalities [3]. EEG measures electrical currents in the dendrites of neurons that are close to the surface of the cerebral cortex with high resolution [4]. Currently, specialist doctors visually extract information from EEG signals to diagnose epileptic seizures. In this process, neurologists can diagnose the condition based on important information in EEG signals, including spikes, sharp waves, and slow waves [5]. Therefore, this method is highly dependent on the experience of doctors specializing in the analysis of EEG signals [5].
Visual analysis of EEG signals is always challenging for neurologists due to the variety of epileptic seizures. Additionally, EEG signals are usually recorded under different conditions, such as with EEG devices that have different sampling frequencies, along with various artifacts from patients. This variability makes it difficult to diagnose epileptic seizures accurately. Misdiagnosis of epileptic seizures by specialist doctors can cause irreparable damage to patients [4, 5]. For example, epileptic seizures are generally classified into two categories: focal and generalized [6]. Misdiagnosis of the type of epileptic seizure can lead to the prescription of inappropriate medications, which may result in drug-resistant epilepsy and, ultimately, death [4, 5]. Therefore, diagnosing epileptic seizures at their early stages from EEG signals is vital for specialist doctors. Over the years, the use of artificial intelligence (AI) techniques, especially machine learning (ML) methods [7, 8] and deep learning (DL) networks [9, 10], to assist in detecting epileptic seizures from EEG signals has grown significantly.
In recent years, extensive research has been conducted in the field of diagnosing epileptic seizures through computer-aided diagnosis systems (CADS) [7-10]. An AI-based CADS consists of a dataset, preprocessing, feature extraction and selection, as well as classification [9, 10]. Feature extraction is the most crucial part of a CADS for detecting epileptic seizures from EEG signals. Until 2016, most researchers focused on CADS utilizing ML techniques [7, 8]. In the field of ML, researchers have presented various methods to extract features from EEG signals aimed at improving the accuracy of diagnosing epileptic seizures. ML feature extraction techniques include time domain, frequency domain, time-frequency domain, and nonlinear transformation methods [3, 4]. In ML-based CADS, researchers often combine features from different domains to enhance the accuracy of epileptic seizure diagnosis. This work is typically performed through trial and error and is highly dependent on the individual’s expertise in the field of ML [3, 4].
With the advent of DL techniques, these networks have quickly replaced ML methods in various medical applications, particularly in the diagnosis of epileptic seizures [9, 10]. Compared to ML techniques, DL networks have achieved promising results in detecting epileptic seizures from EEG data. While DL models offer numerous advantages, they also have some disadvantages, including high computational costs and the need for expensive GPU processors [11]. Nevertheless, researchers are eager to implement DL architectures in the diagnosis of epileptic seizures due to their significant features, such as the ability to automatically extract features from EEG signals [10]. The most important DL architectures used in applications for epileptic seizure detection include convolutional neural networks (CNNs) [12], recurrent neural networks (RNNs) [13], autoencoders (AEs) [14], deep belief networks (DBNs) [15], attention mechanisms [16], and graph models [17]. The development of DL architectures provides hope for researchers, as they aim to implement practical tools for diagnosing epileptic seizures from EEG signals in hospital environments and specialized clinics in the future.
Attention mechanism architectures are a new category of DL techniques that have achieved significant results in the diagnosis of brain disorders [18, 19]. Unlike other DL networks, these networks perform well with limited input data. These architectures focus on specific regions of EEG signals that contain important information, allowing for the extraction of the most critical features from EEG signals. Transformer models represent a new class of attention mechanism networks and are widely used as powerful tools in the analysis of time series, such as EEG signals [20, 21]. In this paper, we presented a new method for diagnosing epileptic seizures based on 1D-CNN Bi-LSTM attention mechanism. First, the EEG signals underwent several preprocessing steps, including filtering, down-sampling, re-referencing using common average reference (CAR), and segmenting the data. Subsequently, the proposed DL architecture was employed to extract features from the preprocessed EEG signals. After that, Sigmoid was utilized to classify the input data, and their results were compared. Finally, the t-distributed stochastic neighbor embedding (t-SNE) technique and shapley additive explanations (SHAP) [25, 26] were used as an explainable artificial intelligence (XAI) technique to represent the feature space.
Related works
In recent years, numerous studies have been conducted in the field of diagnosing epileptic seizures from EEG signals using AI techniques, particularly DL networks. In all these articles, the main goal of the researchers has been to find new DL methods to assist in the early diagnosis of epileptic seizures. Below, several articles that focus on diagnosing epileptic seizures using novel DL models are examined.
Samee et al. utilized a combination of RNN and bidirectional long short-term memory (Bi-LSTM) architectures to detect epileptic seizures [27]. In their work, the Bonn dataset was selected for the simulations. The authors employed EEG signal windowing in the preprocessing step. They then proposed a DL model that fuses RNN with Bi-LSTM to extract features from EEG signals. Finally, they used the Softmax function to classify the extracted features and achieved acceptable results.
In another study, Choi et al. proposed a new DL architecture based on an attention mechanism for diagnosing epileptic seizures [28]. First, EEG signals were preprocessed through filtering, segmentation, and normalization. Subsequently, 1D convolutional neural networks (1D-CNN), gated recurrent units (GRU), and attention mechanism networks were employed for feature extraction and classification. Their proposed DL architecture aimed to extract spatial and temporal features to enhance the accuracy of epileptic seizure diagnosis from EEG signals. Finally, the Softmax activation function was utilized in the last fully connected (FC) layer of the proposed DL architecture for classification.
A novel method for detecting epileptic seizures from EEG signals using a graph convolutional neural network (GCNN) model was introduced by Jia et al. [29]. The preprocessing steps in their work included both low- and high-level processes. Low-level preprocessing involved filtering, normalization, and segmentation. Following this, various features were extracted from EEG signals as part of the high-level preprocessing step. Finally, a GCNN model with the Softmax function was employed for feature extraction and classification, respectively.
In another study, Wang et al. proposed a new DL architecture to detect epileptic seizures [30]. This architecture consists of two dynamic multi-graph convolution networks (DMGCN) and a channel-weighted transformer (CWTr) to extract features from EEG signals. In their research, the CHB-MIT dataset was chosen for the experiments, during which the EEG signals were decomposed into different time windows in the preprocessing step. The proposed DL architecture was then applied in the feature extraction stage. Finally, the Softmax function was used in the classification step, enabling the researchers to achieve satisfactory results.
In another research [31], the authors proposed a method for detecting epileptic seizures from EEG signals using a transformer architecture. In the preprocessing step, normalization and windowing were first applied to the EEG signals of the CHB-MIT dataset. Then, the EEG signals were converted into 2D images using the short-time fourier transform (STFT) method. Next, feature extraction from the 2D images was performed using a hybrid transformer model. Finally, in the classification step, the Softmax algorithm was employed in the last FC layer of the proposed DL architecture.
Jibon et al. introduced a graph deep learning (GDL) architecture to extract features from EEG signals [32]. In this work, simulations were conducted on the TUH and CHB-MIT datasets. The preprocessing of EEG signals includes both low-level and high-level components. Low-level preprocessing involves filtering and windowing, while high-level preprocessing includes EEG signal decomposition, feature extraction, and graph representation. Subsequently, sequential graph convolutional network (SGCN) and RNN architectures were utilized for the feature extraction and classification stages.
In another study, researchers presented a method for diagnosing epileptic seizures using dynamic brain functional connectivity [33]. In this work, the TUH dataset was first selected for experiments. Next, EEG signals were preprocessed using a functional connectivity method to convert the EEG signals into 2D images. They then employed a graph-generative neural network (GGNN) architecture to extract features from the 2D functional connectivity images. Finally, the Softmax function was applied to classify the input features.
A new method for detecting epileptic seizures based on a linear graph convolutional network (LGCN) and DenseNet was presented in another study [17]. In the preprocessing step, the authors separated the EEG signals into different time intervals. Following this, they applied the Stockwell transform and graph structure methods to the EEG window signals. Next, LGCN and DenseNet architectures were integrated and employed for feature extraction and classification. The results of this work demonstrated that the authors achieved significant success in diagnosing epileptic seizures.
Ma et al. [34] extracted temporal and spatial features using a proposed DL architecture for the detection of epileptic seizures. In this study, the CHB-MIT and UCI datasets were selected, after which preprocessing steps, including normalization and one-hot encoding, were applied. The proposed DL architecture consists of 1D-CNN and Bi-LSTM blocks with an attention mechanism to extract features from EEG signals. This part is capable of extracting both spatial and temporal features simultaneously. In this work, the authors achieved acceptable accuracy in diagnosing epileptic seizures.
In another study, Lih et al. [35] employed a transformer architecture with the aim of improving the diagnosis of epileptic seizures. In this research, they presented a new dataset with 121 subjects, including two classes: seizure and healthy control (HC). In the preprocessing step, the EEG signals were first segmented into different time windows, after which the Pearson correlation coefficient (PCC) was calculated for each EEG segment. Next, feature extraction and classification steps were performed using a proposed transformer architecture on the 2D PCC images. The most significant innovation of this research lies in the data section.
Methods
In this section, the proposed method for diagnosing epileptic seizures based on EEG signals is presented. In Figure 1, details of the proposed CADS sections based on a new DL network are shown. As illustrated, the proposed method consisted of dataset preparation, preprocessing, feature extraction, classification, and post-processing sections. The simulation of the proposed method was performed using the Turkish epilepsy EEG dataset, which included 121 subjects divided into two classes: epileptic seizures and HC [35]. Preprocessing steps, including filtering, down-sampling, re-referencing using CAR, and segmentation, were applied to the EEG signals. In the next step, a proposed DL architecture, which consists of a 1D-CNN Bi-LSTM fused with an attention mechanism, was employed to extract spatio-temporal features from the preprocessed EEG signals. EEG signals are non-linear and spatio-temporal in nature [36-38]. The 1D-CNN architecture extracts spatial features from the EEG signals, while the Bi-LSTM network captures temporal dependencies. The attention mechanism helps the model focus on the most relevant parts of the signal, thereby increasing the accuracy of diagnosing epileptic seizures from EEG signals. Finally, t-SNE and SHAP were utilized as an XAI technique in the post-processing step to visualize the feature space extracted by the proposed DL architecture [25, 26].
Dataset and preprocessing
As mentioned, the Turkish epilepsy dataset was selected to perform simulations and assess the performance of the proposed DL architecture. In this dataset, EEG data were recorded from 121 subjects, of whom 50 experienced epileptic seizures while 71 were classified as HC [35]. EEG signals were recorded from the parietal, frontal, temporal, occipital, frontopolar, auricular, and central regions. Data recording was conducted using the 10-20 electrode placement standard, with 35 channels and a sampling frequency of 500 Hz [35]. In this section, first, a band-pass filter (0.5–48 Hz) was applied to eliminate artifacts and retain frequency components of interest. Further, the EEG signals were down-sampled to 250 Hz. CAR was then used to enhance the signal-to-noise ratio by subtracting the mean activities across all channels from each individual channel; this would suppress common noises. Finally, the preprocessed EEG signals were divided into non-overlapping windows of 5 s, 10 s, and 15 s duration.
DL model
Today, DL architectures are increasingly being used in the field of diagnosing epileptic seizures from EEG signals. 1D-CNNs are an important category of DL models that effectively extract spatial features from EEG signals; however, they overlook the temporal correlations between EEG channels [36, 37]. RNNs represent another category of DL frameworks that are widely used for feature extraction from EEG signals. RNN models are generally more successful at capturing temporal features compared to CNN models [21]. Researchers have demonstrated that CNN-RNN architectures can successfully extract both spatial and temporal features from EEG signals. However, these architectures lack a mechanism to identify the importance of specific parts of the input EEG signals, such as critical channels, which could improve the accuracy of epileptic seizure detection. Consequently, CNN-RNN architectures frequently face challenges in the simultaneous extraction of spatio-temporal features. To address this issue, attention mechanism architectures have been introduced to overcome the limitations of RNN models in feature extraction from EEG signals. In this paper, we proposed an improved architecture based on the 1D-CNN Bi-LSTM Attention to extract both spatial and temporal features from EEG signals.
1D-CNN module
The presented model initially used 1D-CNN blocks, which are responsible for extracting spatial features from EEG raw signals. The basic operations inside these convolutional blocks were formally described as follows (Equation 1):
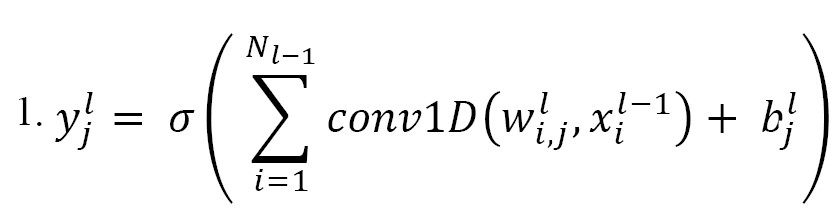
where denotes the i-th feature map in the (l − 1)-th layer; denotes the j-th feature map in the l-th layer, while represents the trainable convolutional kernel; represents the number of feature maps in the (l-1)-th layer; conv1D represents the 1D convolution operation without zero-padding; represents the bias of the j-th feature map in the l-th layer; and denotes the ReLU activation function, which is used to suppress overfitting and increase the non-linearity of the model. It is defined as follows (Equation 2):
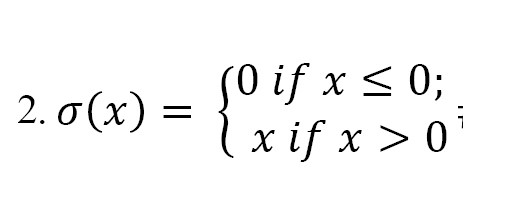
The max-pooling operation in layer was defined as follows (Equation 3):
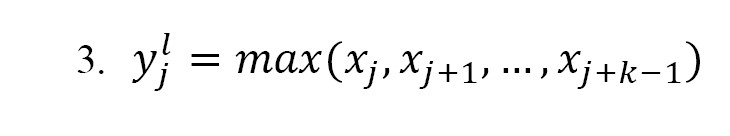
In this operation, a sliding window of size k was applied over the input sequence. For each position j, the max-pooling function selects the maximum value within the window. Max-pooling contained no trainable parameters and its purpose was to reduce the dimensionality of the feature representation.
Table 1 summarizes the specifications of the 1D-CNN Bi-LSTM Attention architecture, which comprises a total of 17 layers.

The input data first passes through a 1D convolutional layer with 64 filters, a kernel size of 3, a stride of 1, and ReLU for activation. This was followed by a max-pooling layer to reduce the dimensionality by half, and a dropout layer with a rate of 0.5 was used to control overfitting. Next, another 1D convolutional layer with 48 filters, a kernel size of 3, a stride of 1, and ReLU for activation was used. Similar to the previous block, this layer is followed by a max-pooling layer and a dropout layer with a rate of 0.5. A third convolutional layer, containing 32 filters, a kernel size of 3, a stride of 1, and ReLU for activation, is then used; this is again followed by a max-pooling layer with a pool size of 2. These convolutional, pooling, and dropout operations extracted hierarchical spatial features in the EEG signals while controlling overfitting. The output of the last block was fed into the next subsequent modules of the network for processing.
Bi-LSTM module
After the 1D-CNN feature extraction, the output feature sequences were utilized by a Bi-LSTM network, which is able to identify the temporal dynamics of the EEG signals. The main operation of the Bi-LSTM network is based on the functions performed inside the LSTM unit. The activities that happen in the main LSTM unit at time are mathematically described below (Equation 4):
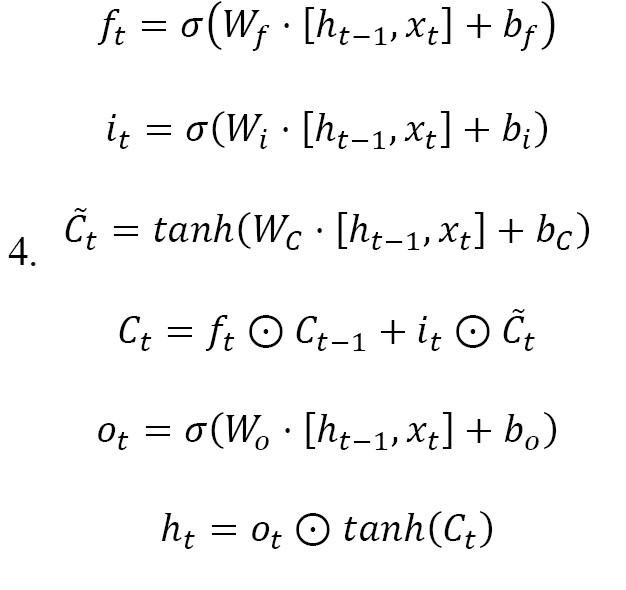
Where xt symbolizes the input feature vector, ht-1 indicates the prior hidden state, ct-1 represents the prior cell state, σ is the Sigmoid function, and is the Hadamard product. The Bi-LSTM architecture processes the input in both forward ht and backward ht directions, enabling the model to effectively learn long-range dependencies in the temporal domain. Each time step is passed through two parallel LSTM units—one moving from past to future and the other from future to past—providing a richer representation of temporal patterns. The Bi-LSTM has 32 hidden units in both directions. The final output vector Ht of the Bi-LSTM layer is the concatenation of the forward and backward hidden states (Equation 5):
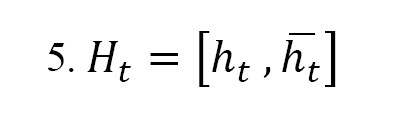
Attention mechanism module
The Bi-LSTM layer generates activation vectors that will be input into an attention mechanism built to help accentuate the most relevant temporal variables. The mathematical formulation of the attention mechanism is defined as follows, where is the output matrix of the Bi-LSTM (Equation 6):
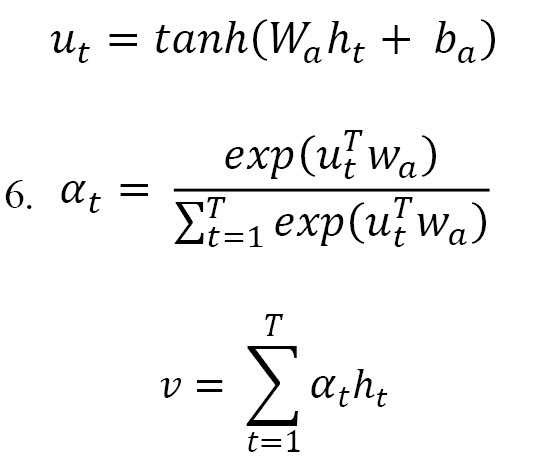
The attention layer produced a distribution of attention weights that favor the time steps most applicable to the classification task. This was accomplished by computing all of the Bi-LSTM outputs ht and multiplying them by their associated attention weight, allowing the Bi-LSTM to dynamically adjust the focus of learning on the relevant temporal components of the data and filter out any noise or otherwise unhelpful data. A Global Average Pooling 1D layer then aggregated these weighted representations into a compact feature vector, which was subsequently forwarded to the FC layers for final classification.
Experiments
Hardware and software resources
In this section, we reported the results of the proposed DL architecture for epileptic seizure detection from EEG signals. All simulations in this study were conducted using a hardware system equipped with an NVIDIA 1070 GPU, 512 GB of RAM, and a Core i7 CPU. Additionally, TensorFlow [40] and Scikit-learn tools [40] were used for implementing the DL architecture and calculating the evaluation metrics, respectively. The implementation of the t-SNE technique [25] was also carried out using the Scikit-learn library [40].
Evaluation metrics
Calculating evaluation metrics is essential for evaluating the performance of the proposed DL architecture for epileptic seizure diagnosis. In this section, evaluation measures, including accuracy (Acc), sensitivity (Sen), specificity (Spec), precision (Prec), and F1 score (F1) were calculated. The formulas for these evaluation metrics are briefly reported in Table 2.

To implement the proposed DL model, 5-fold cross-validation was applied to the entire EEG dataset. In each fold, 80% of the data was used for training and the remaining 20% served as the test set. After that, the aforementioned evaluation metrics were calculated for the training, testing, and validation data. For this purpose, the Scikit-learn library [40] was employed to compute the evaluation metrics in a Python environment. Given the importance of data validation, this study reported the results of validation data in the experimental results section.
Implementation details
The hyperparameters of the DL network proposed to achieve optimal performance are presented below. The number of epochs, batch size, and regularization (L1) were set at 100, 32, and 0.0009, respectively. Furthermore, the Adam optimization method and binary cross-entropy were utilized as the cost function in the implementation of the proposed DL network. Additionally, the Sigmoid activation function was employed as the classification step.
Results
This section presents the results of the proposed DL architecture for detecting epileptic seizures from EEG signals. Tables 3, 4 and 5 show the performance of different DL architectures applied to EEG signals with various time windows. For each EEG time frame, the results of the 1D-CNN, 1D-CNN Bi-LSTM, and 1D-CNN Bi-LSTM attention models were compared.



According to Tables 3, 4 and 5, the proposed 1D-CNN Bi-LSTM attention architecture with a 10-second frame achieved the highest accuracy in detecting epileptic seizures.
Accordingly, additional results for the proposed DL architecture with a 10-second frame are presented below. Figure 2 illustrates the ROC curve and confusion matrix of the proposed 1D-CNN Bi-LSTM attention model based on EEG signals with a 10-second time frame. As shown, the proposed DL architecture achieved strong performance in epileptic seizure detection.
Next, the results of XAI methods, including t-SNE and SHAP models, are presented to provide further insights into the proposed method [25, 26]. By reducing high-dimensional EEG data to a lower-dimensional space, t-SNE facilitates the visualization of complex patterns and relationships in seizure data, enabling clinicians to understand the fundamental features that distinguish different types of seizures. This interpretability is critical for practitioners, as it provides insights into the model’s decision-making process and allows for a more informed evaluation of the classifications produced by the proposed DL model. Figure 3 displays the results of the t-SNE method for the proposed DL architecture based on 5-fold cross-validation technique. The interpretability of the proposed model was assessed using SHAP, which is a unified framework to explain predictions based on cooperative game theory. SHAP assigns a unique importance value to each feature, representing its average marginal contribution to the model’s prediction across all possible feature combinations. This, in turn, helps us understand the local behavior of which specific features drive individual classification decision [26]. The results of the SHAP model as an XAI technique are shown in Figure 4. Figure 4a presents the SHAP summary bar plot, which displays the absolute mean SHAP values for each feature. This identifies the most influential EEG channels—PZA2, F3C3, P4A2, C3P3, and T3T5—in detecting epileptic seizures using the proposed DL model. These channels were located in the frontal, temporal, and parietal regions, which is consistent with neurophysiological evidence. Figure 4b also shows the SHAP Beeswarm plot, which illustrates the full distribution of SHAP values for each feature and indicates how increases or decreases in feature values affect the probability of seizure detection. The color of the dots represents feature values (blue for low, red for high). For important features, such as PZA2, F3C3, and P4A2, high feature values (red points) generally correspond to positive SHAP values, thereby increasing the likelihood of seizure prediction. This behavior aligns with typical EEG patterns during seizures, including increased amplitude, sharp waves, and burst activity.
Discussion
Epileptic seizures are among the most well-known neurological disorders caused by abnormal electrical discharges in brain neurons [2-4]. This condition is associated with transient seizures throughout the day and poses serious health risks to patients, including fainting, anesthesia, and loss of muscle control [1-3]. Generally, neurologists diagnose epileptic seizures by examining abnormal amplitudes on EEG waveforms, a task that is very time-consuming and associated with human error [7, 8]. Because EEG signals are non-linear and contain various artifacts, their visual analysis is challenging for expert clinicians. Additionally, EEG signals are usually recorded over long periods and with different sampling frequencies, making accurate visual observation extremely time-consuming for doctors [9-11]. Moreover, EEG signals are captured through multiple channels, further complicating the data used for diagnosing epileptic seizures.
In recent years, AI techniques, particularly DL models, have garnered attention from researchers for diagnosing epileptic seizures from EEG signals [9, 10]. This study introduced a new method for diagnosing epileptic seizures based on a 1D-CNN Bi-LSTM attention architecture. The proposed method encompassed several sections: dataset preparation, preprocessing, feature extraction, classification, and post-processing. The implementation and evaluation of this method were conducted using a Turkish epilepsy dataset [35]. Initially, preprocessing steps, such as band-pass filtering (0.5–48 Hz), down-sampling to 250 Hz, re-referencing using CAR, and segmenting were applied to the EEG signals. Subsequently, the proposed DL model was implemented for extracting spatial-temporal features from the preprocessed EEG signals. Finally, the t-SNE method and SHAP [25, 26] were employed as a post-processing step to visualize the space of the extracted features. In Table 6, we presented the results of papers on diagnosis of epileptic seizures and compared them with our proposed method.

As observed, our proposed method demonstrated significant results compared to other research.
In the future, the proposed method could serve as practical software in hospital settings to assist specialist doctors in the rapid diagnosis of epileptic seizures based on EEG signals. Recently, some researchers have utilized new attention mechanism architectures, especially attention-graph models [48, 49] and transformer models with mutual learning architectures [50, 51], in medical applications. For future work, these DL networks could be applied to the diagnosis of epileptic seizures. It has been demonstrated that Graph DL networks have been highly successful in diagnosing brain disorders from EEG signals [52, 53]. For further work, the use of new graph architectures, such as multi-layer graph attention networks (MGANet) [54], spatial-temporal graph attention networks with transformer encoders (STGATE) [55], and adaptive gated graph convolutional networks (AGGCN) [56] should be considered for detecting epileptic seizures.
Conclusion
In conclusion, epileptic seizures pose significant health challenges and are traditionally diagnosed through time-consuming and error-prone EEG analysis. The integration of advanced deep learning models, such as the proposed 1D-CNN Bi-LSTM attention architecture, demonstrates promising improvements in accurately detecting seizures from EEG signals. The study’s results indicate that this approach outperforms existing methods, highlighting its potential for clinical application. Future research should explore incorporating cutting-edge attention mechanisms and graph-based neural networks to further enhance diagnostic accuracy and facilitate practical deployment in medical settings.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Data extraction and Simulation: Mohammad Mehdi Barzegar; Review and editing: Mohammad Mehdi Barzegar and Marjane Khodatars; Conceptualization, supervision, and writing the original draft: All authors.
Conflict of interest
The authors declared no conflicts of interest.
References
- Rasheed K, Qayyum A, Qadir J, Sivathamboo S, Kwan P, Kuhlmann L, et al. Machine learning for predicting epileptic seizures using EEG signals: A review. IEEE Reviews in Biomedical Engineering. 2020; 14:139-55. [DOI:10.1109/RBME.2020.3008792] [PMID]
- Dash DP, Kolekar M, Chakraborty C, Khosravi MR. Review of machine and deep learning techniques in epileptic seizure detection using physiological signals and sentiment analysis. ACM Transactions on Asian and Low-Resource Language Information Processing. 2024; 23(1):1-29. [DOI:10.1145/3552512]
- Shoeibi A, Khodatars M, Ghassemi N, Jafari M, Moridian P, Alizadehsani R, et al. Epileptic seizures detection using deep learning techniques: A review. International Journal of Environmental Research and Public Health. 2021; 18(11):5780. [DOI:10.3390/ijerph18115780] [PMID]
- Shoeibi A, Ghassemi N, Alizadehsani R, Rouhani M, Hosseini-Nejad H, Khosravi A, et al. A comprehensive comparison of handcrafted features and convolutional autoencoders for epileptic seizures detection in EEG signals. Expert Systems with Applications. 2021; 163:113788. [DOI:10.1016/j.eswa.2020.113788]
- Abdulwahhab AH, Abdulaal AH, Al-Ghrairi AHT, Mohammed AA, Valizadeh M. Detection of epileptic seizure using EEG signals analysis based on deep learning techniques. Chaos, Solitons & Fractals. 2024; 181:114700. [DOI:10.1016/j.chaos.2024.114700]
- Ahmad I, Wang X, Zhu M, Wang C, Pi Y, Khan JA, et al. EEG‐Based Epileptic Seizure Detection via Machine/Deep Learning Approaches: A systematic review. Computational Intelligence and Neuroscience. 2022; (1):6486570. [DOI:10.1155/2022/6486570] [PMID]
- Si Y. Machine learning applications for electroencephalograph signals in epilepsy: A quick review. Acta Epileptologica. 2020; 2(1):5. [DOI:10.1186/s42494-020-00014-0]
- Miltiadous A, Tzimourta KD, Giannakeas N, Tsipouras MG, Glavas E, Kalafatakis K, et al. Machine learning algorithms for epilepsy detection based on published EEG databases: A systematic review. IEEE Access. 2022; 11:564-94. [DOI:10.1109/ACCESS.2022.3232563]
- Usman SM, Khalid S, Aslam MH. Epileptic seizures prediction using deep learning techniques. Ieee Access. 2020; 8:39998-40007. [DOI:10.1109/ACCESS.2020.2976866]
- Xu J, Yan K, Deng Z, Yang Y, Liu JX, Wang J, Yuan S. EEG-based Epileptic Seizure Detection using Deep Learning Techniques: A Survey. Neurocomputing. 2024; 128644. [DOI:10.1016/j.neucom.2024.128644]
- Jafari M, Sadeghi D, Shoeibi A, Alinejad-Rokny H, Beheshti A, García DL, et al. Empowering precision medicine: AI-driven schizophrenia diagnosis via EEG signals: A comprehensive review from 2002-2023. Applied Intelligence. 2024; 54(1):35-79. [DOI:10.1007/s10489-023-05155-6]
- Hu Y, Liu J, Sun R, Yu Y, Sui Y. Classification of epileptic seizures in EEG data based on iterative gated graph convolution network. Frontiers in Computational Neuroscience. 2024; 18:1454529. [DOI:10.3389/fncom.2024.1454529] [PMID]
- Rukhsar S, Tiwari AK. ARNN: Attentive Recurrent Neural Network for Multi-channel EEG Signals to Identify Epileptic Seizures. arXiv preprint arXiv:2403.03276. 2024. [DOI:10.1016/j.neucom.2024.129203]
- Liang S, Zhang X, Zhao H, Dang Y, Hui R, Zhang J. Double Discrete Variational Autoencoder for Epileptic EEG Signals Classification. IEEE Access. 2024. [DOI:10.1109/ACCESS.2024.3429195]
- Movahedi F, Coyle JL, Sejdić E. Deep belief networks for electroencephalography: A review of recent contributions and future outlooks. IEEE Journal of Biomedical and Health Informatics. 2017; 22(3):642-52. [DOI:10.1109/JBHI.2017.2727218] [PMID]
- Xin Q, Hu S, Liu S, Zhao L, Zhang YD. An attention-based wavelet convolution neural network for epilepsy EEG classification. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2022; 30:957-66. [DOI:10.1109/TNSRE.2022.3166181] [PMID]
- Jibon FA, Miraz MH, Khandaker MU, Rashdan M, Salman M, Tasbir A, et al. Epileptic seizure detection from electroencephalogram (EEG) signals using linear graph convolutional network and DenseNet based hybrid framework. Journal of Radiation Research and Applied Sciences. 2023; 16(3):100607. [DOI:10.1016/j.jrras.2023.100607]
- Khan AA, Madendran RK, Thirunavukkarasu U, Faheem M. D2PAM: epileptic seizures prediction using adversarial deep dual patch attention mechanism. CAAI Transactions on Intelligence Technology. 2023; 8(3):755-69. [DOI:10.1049/cit2.12261]
- Huang H, Chen P, Wen J, Lu X, Zhang N. Multiband seizure type classification based on 3D convolution with attention mechanisms. Computers in Biology and Medicine. 2023; 166:107517. [DOI:10.1016/j.compbiomed.2023.107517] [PMID]
- Shoeibi A, Khodatars M, Alinejad-Rorky H, Heras J, Bagherzadeh S, Beheshti A, et al. Diagnosis of schizophrenia in EEG signals using dDTF effective connectivity and new pretrained CNN and transformer models. In: International Work-Conference on the Interplay Between Natural and Artificial Computation. Cham: Springer Nature Switzerland; 2024. [DOI:10.1007/978-3-031-61140-7_15]
- Shoeibi A, Jafari M, Sadeghi D, Alizadehsani R, Alinejad-Rokny H, Beheshti A, et al. Early diagnosis of schizophrenia in EEG signals using one dimensional transformer model. In: International Work-Conference on the Interplay Between Natural and Artificial Computation. Cham: Springer Nature Switzerland; 2024. [DOI:10.1007/978-3-031-61140-7_14]
- Biau G, Scornet E. (2016). A random forest guided tour. Test. 2016; 25:197-227. [DOI:10.1007/s11749-016-0488-0]
- Kotsiantis, SB. Decision trees: a recent overview. Artificial Intelligence Review. 2013; 39:261-83. [DOI:10.1007/s10462-011-9272-4]
- Hastie T, Rosset S, Zhu J, Zou H. Multi-class adaboost. Statistics and Its Interface. 2009; 2(3):349-60. [DOI:10.4310/SII.2009.v2.n3.a8]
- Van der Maaten L, Hinton G. Visualizing data using t-SNE. Journal of Machine Learning Research. 2008; 9(11):2580-605. [Link]
- Lundberg SM, Lee SI. A unified approach to interpreting model predictions. Advances in neural information processing systems. Paper presented at: 31st Conference on Neural Information Processing Systems (NIPS 2017). 4 December 2017; Long Beach, USA. [Link]
- Samee NA, Mahmoud NF, Aldhahri EA, Rafiq A, Muthanna MSA, Ahmad I. RNN and Bi-LSTM fusion for accurate automatic epileptic seizure diagnosis using EEG signals. Life. 2022; 12(12):1946. [DOI:10.3390/life12121946] [PMID]
- Choi W, Kim MJ, Yum MS, Jeong DH. Deep convolutional gated recurrent unit combined with attention mechanism to classify pre-ictal from interictal EEG with minimized number of channels. Journal of Personalized Medicine. 2022; 12(5):763. [DOI:10.3390/jpm12050763] [PMID]
- Jia M, Liu W, Duan J, Chen L, Chen CP, Wang Q, et al. Efficient graph convolutional networks for seizure prediction using scalp EEG. Frontiers in Neuroscience. 2022; 16:967116. [DOI:10.3389/fnins.2022.967116] [PMID]
- Wang Y, Cui W, Yu T, Li X, Liao X, Li Y. Dynamic multi-graph convolution based channel-weighted transformer feature fusion network for epileptic seizure prediction. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2023. [DOI:10.1109/TNSRE.2023.3321414]
- Hu S, Liu J, Yang R, Wang YN, Wang A, Li K, et al. Exploring the applicability of transfer learning and feature engineering in epilepsy prediction using hybrid transformer model. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2023; 31:1321-32. [DOI:10.1109/TNSRE.2023.3244045] [PMID]
- Jibon FA, Jamil Chowdhury AR, Miraz MH, Jin HH, Khandaker MU, Sultana S, et al. Sequential graph convolutional network and DeepRNN based hybrid framework for epileptic seizure detection from EEG signal. Digital Health. 2024; 10:20552076241249874. [DOI:10.1177/20552076241249874] [PMID]
- Li Z, Hwang K, Li K, Wu J, Ji T. Graph-generative neural network for EEG-based epileptic seizure detection via discovery of dynamic brain functional connectivity. Scientific Reports. 2022; 12(1):18998. [DOI:10.1038/s41598-022-23656-1] [PMID]
- Ma Y, Huang Z, Su J, Shi H, Wang D, Jia S, et al. A multi-channel feature fusion CNN-BI-LSTM epilepsy EEG classification and prediction model based on attention mechanism. IEEE Access. 2023; 11:62855-64. [DOI:10.1109/ACCESS.2023.3287927]
- Lih OS, Jahmunah V, Palmer EE, Barua PD, Dogan S, Tuncer T, et al. EpilepsyNet: Novel automated detection of epilepsy using transformer model with EEG signals from 121 patient population. Computers in Biology and Medicine. 2023; 164:107312. [DOI:10.1016/j.compbiomed.2023.107312] [PMID]
- Bagherzadeh S, Shalbaf A, Shoeibi A, Jafari M, Tan RS, Acharya UR. Developing an EEG-based emotion recognition using ensemble deep learning methods and fusion of brain effective connectivity maps. IEEe Access. 2024; 12:50949-65. [DOI:10.1109/ACCESS.2024.3384303]
- Bdaqli M, Shoeibi A, Moridian P, Sadeghi D, Pouyani MF, Shalbaf A, et al. Diagnosis of Parkinson disease from EEG signals using a CNN-LSTM model and explainable AI. In International work-conference on the interplay between natural and artificial computation. Cham: Springer Nature Switzerland; 2024. [DOI:10.1007/978-3-031-61140-7_13]
- Nazari R, Salehi M, Shoeibi A. An Explainable Connectome Convolutional Transformer for Multimodal Autism Spectrum Disorder Classification. International Journal of Neural Systems. 2025; 35(8):2550043-2550043. [DOI:10.1142/S0129065725500431] [PMID]
- Weber M, Wang H, Qiao S, Xie J, Collins MD, Zhu Y, et al. Deeplab2: A tensorflow library for deep labeling. arXiv preprint arXiv:2106.09748 [Unpublished]. [Link]
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine learning in Python. The Journal of Machine Learning Research. 2011; 12:2825-30. [Link]
- He J, Cui J, Zhang G, Xue M, Chu D, Zhao Y. Spatial-temporal seizure detection with graph attention network and bi-directional LSTM architecture. Biomedical Signal Processing and Control. 2022; 78:103908. [DOI:10.1016/j.bspc.2022.103908]
- Chanu MM, Singh NH, Thongam K. An automated epileptic seizure detection using optimized neural network from EEG signals. Expert Systems. 2023; 40(6):e13260. [DOI:10.1111/exsy.13260]
- Srinivasan S, Dayalane S, Mathivanan SK, Rajadurai H, Jayagopal P, Dalu GT. Detection and classification of adult epilepsy using hybrid deep learning approach. Scientific Reports. 2023; 13(1):17574. [DOI:10.1038/s41598-023-44763-7] [PMID]
- Tripathi PM, Kumar A, Kumar M, Komaragiri RS. Automatic seizure detection and classification using super-resolution superlet transform and deep neural network-A preprocessing-less method. Computer Methods and Programs in Biomedicine. 2023; 240, 107680. [DOI:10.1016/j.cmpb.2023.107680] [PMID]
- Zhang Y, Yao S, Yang R, Liu X, Qiu W, Han L, et al. Epileptic seizure detection based on bidirectional gated recurrent unit network. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2022; 30:135-45. [DOI:10.1109/TNSRE.2022.3143540] [PMID]
- Shyu KK, Huang SC, Lee LH, Lee PL. Less parameterization inception-based end to end CNN model for EEG seizure detection. IEEE Access. 2023. [DOI:10.1109/ACCESS.2023.3277634]
- Cimr D, Fujita H, Tomaskova H, Cimler R, Selamat A. Automatic seizure detection by convolutional neural networks with computational complexity analysis. Computer Methods and Programs in Biomedicine. 2023; 229:107277. [DOI:10.1016/j.cmpb.2022.107277] [PMID]
- Yan H, Guo K, Xing X, Xu X. Bridge Graph Attention based Graph Convolution Network with Multi-Scale Transformer for EEG Emotion Recognition. IEEE Transactions on Affective Computing. 2024. [DOI:10.1109/TAFFC.2024.3394873]
- Chen T, Guo Y, Hao S, Hong R. Exploring self-attention graph pooling with EEG-based topological structure and soft label for depression detection. IEEE Transactions on Affective Computing. 2022; 13(4):2106-18. [DOI:10.1109/TAFFC.2022.3210958]
- Peng R, Du Z, Zhao C, Luo J, Liu W, Chen X, et al. Multi-Branch Mutual-Distillation Transformer for EEG-Based Seizure Subtype Classification. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2024. [DOI:10.1109/TNSRE.2024.3365713]
- Yi Y, Xu Y, Yang B, Tian Y. A weighted co-training framework for emotion recognition based on EEG data generation using frequency-spatial diffusion transformer. IEEE Transactions on Affective Computing. 2024. [DOI:10.1109/TAFFC.2024.3395359]
- Dissanayake T, Fernando T, Denman S, Sridharan S, Fookes C. Geometric deep learning for subject independent epileptic seizure prediction using scalp EEG signals. IEEE Journal of Biomedical and Health Informatics. 2021; 26(2):527-38. [DOI:10.1109/JBHI.2021.3100297] [PMID]
- Hou Y, Jia S, Lun X, Hao Z, Shi Y, Li Y, et al. GCNs-net: a graph convolutional neural network approach for decoding time-resolved eeg motor imagery signals. IEEE Transactions on Neural Networks and Learning Systems. 2022; 35(6):7312-23. [DOI:10.1109/TNNLS.2022.3202569]
- Zhang W, Yin Z, Sheng Z, Li Y, Ouyang W, Li X, et al. (2022, August). Graph attention multi-layer perceptron. Proceedings of the 28th ACM SIGKDD Conference on Knowledge Discovery and Data Mining. 2022; 4560-70.[DOI:10.1145/3534678.3539121]
- Wu D, Peng K, Wang S, Leung VC. Spatial-Temporal Graph Attention Gated Recurrent Transformer Network for Traffic Flow Forecasting. IEEE Internet of Things Journal. 2023. [DOI:10.1109/JIOT.2023.3340182]
- Klepl D, He F, Wu M, Blackburn DJ, Sarrigiannis P. Adaptive gated graph convolutional network for explainable diagnosis of Alzheimer’s disease using EEG data. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2023. [DOI:10.1109/TNSRE.2023.3321634] [PMID]
Type of Study: Orginal Article |
Subject:
● Artificial Intelligence
Received: 2025/11/20 | Accepted: 2025/11/29 | Published: 2025/12/31
Received: 2025/11/20 | Accepted: 2025/11/29 | Published: 2025/12/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |