Volume 14, Issue 4 (Jul & Aug 2024)
J Research Health 2024, 14(4): 387-394 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Salehi M, Solati A, Bahari P, Sharifan M, Valizadeh T, Shoraka H. Aflatoxin M1 Contamination in Milk From North Khorasan Province: Raw vs Pasteurized vs Sterilized. J Research Health 2024; 14 (4) :387-394
URL: http://jrh.gmu.ac.ir/article-1-2397-en.html
URL: http://jrh.gmu.ac.ir/article-1-2397-en.html
Mitra Salehi1 

 , Akbar Solati2
, Akbar Solati2 

 , Pezhman Bahari3
, Pezhman Bahari3 
 , Mahyar Sharifan4
, Mahyar Sharifan4 
 , Touhid Valizadeh4
, Touhid Valizadeh4 
 , Hamidreza Shoraka5
, Hamidreza Shoraka5 



 , Akbar Solati2
, Akbar Solati2 

 , Pezhman Bahari3
, Pezhman Bahari3 
 , Mahyar Sharifan4
, Mahyar Sharifan4 
 , Touhid Valizadeh4
, Touhid Valizadeh4 
 , Hamidreza Shoraka5
, Hamidreza Shoraka5 

1- Vector-Borne Diseases Research Center, North Khorasan University of Medical Sciences, Bojnourd, Iran.
2- Department of English, School of Medicine, North Khorasan University of Medical Sciences, Bojnourd, Iran.
3- Department of Microbiology, Central Laboratory, North Khorasan Veterinary Organization, Bojnourd, Iran. ,pejmanbahari@gmail.com
4- Department of Microbiology, Central Laboratory, North Khorasan Veterinary Organization, Bojnourd, Iran.
5- Department of Epidemiology, Esfarayen Faculty of Medical Sciences, Esfarayen, Iran.
2- Department of English, School of Medicine, North Khorasan University of Medical Sciences, Bojnourd, Iran.
3- Department of Microbiology, Central Laboratory, North Khorasan Veterinary Organization, Bojnourd, Iran. ,
4- Department of Microbiology, Central Laboratory, North Khorasan Veterinary Organization, Bojnourd, Iran.
5- Department of Epidemiology, Esfarayen Faculty of Medical Sciences, Esfarayen, Iran.
Full-Text [PDF 964 kb]
(313 Downloads)
| Abstract (HTML) (954 Views)
Full-Text: (324 Views)
Introduction
ycotoxins are natural contaminants that can be found in food and agricultural products. They are created by molds and fungi, with certain types of fungi from the Aspergillus genus as the main culprits. Specifically, Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius are known to produce these toxins. According to the Food and Agriculture Organization (FDA), around 25% of agricultural products worldwide are affected by mycotoxin contamination [1]. Fungi tend to thrive in hot and humid environments and can produce mycotoxins. Among the mycotoxins identified in nature, at least 18 types of aflatoxins are known, with aflatoxin B1, B2, G1, and G2 being particularly significant. When animals consume feed contaminated with aflatoxin B1, the toxin is metabolized in the liver and converted into aflatoxin M1 (AFM1). This mycotoxin is then excreted in milk and can harm human organs, particularly the digestive system and liver. Depending on its concentration and duration, AFM1 can cause mutagenesis, carcinogenesis, and immune system suppression [2]. Meanwhile, AFM1 resists common heat treatment methods like pasteurization, sterilization, and autoclaving [3]. AFM1’s high resistance to freezing and fermentation makes it a concern in producing raw animal products like yogurt, buttermilk, and cheese, which can be made using contaminated milk [4]. Although children are more susceptible to the health risks associated with AFM1-contaminated milk, dairy products are also important sources of nutrients essential for human growth and well-being, especially in children [5]. The International Agency for Research on Cancer recently classified aflatoxin M1 as a Group 1 carcinogen, highlighting the significant health risks this mycotoxin poses [6]. To safeguard the safety and health of human societies, government institutions must conduct rigorous monitoring of food and livestock products. To mitigate the adverse effects of AFM1, many countries and international organizations have established maximum permissible limits for this mycotoxin in milk and dairy products. Various chromatography techniques, including thin-layer chromatography, liquid chromatography, high-performance liquid chromatography, and enzyme-linked immunosorbent assay (ELISA), are available for detecting and analyzing AFM1 in food, milk, and dairy products. ELISA is a particularly advantageous method, offering reduced test times, simple sample preparation and extraction, low cost, and high sensitivity [7]. Several studies have investigated the levels of AFM1 in raw and pasteurized milk in Iran, including in North Khorasan Province, Iran; however, a comprehensive study of this issue has yet to be conducted in the region [7-10]. Given that traditional livestock management practices are common in the province and may not prioritize health standards, it is necessary to implement specific management measures and prevention programs. Therefore, this study assesses the levels of AFM1 in raw, pasteurized, and sterilized milk produced in the cities within North Khorasan Province, Iran.
Methods
This study is a descriptive-analytical investigation conducted in North Khorasan Province, Iran. The minimum sample size was calculated using the method described by Karimi et al. in 2007, considering a contamination rate of 60% (P=0.6), a confidence level of 95%, and an acceptable error value of 5%, total sample size calculated was 258 [11] (Equation 1):

The samples were divided into two groups as follows: Raw and pasteurized milk. The sampling method in this study is multi-stage. First, we consider each city as a stratum, and sampling is done from all strata. In the next stage of sampling, the cluster sampling method was used, each industrial livestock unit was considered a cluster, and each unit of milk collection center and milk tanker was taken as a cluster. In addition, each traditional livestock was considered a cluster. Based on the number of traditional livestock in each village, the number of samples was determined. A total of 189 raw milk samples were collected randomly in 6 months from various sources in the cities of Bojnurd, Esfarayen, Shirvan, Jajarm, Mane and Samalqan, Garmeh, Faruj, and Raz and Jargalan. This study was conducted in the summer and autumn of 2022. These seasons are suitable for the growth of molds and fungi. The samples were obtained from 39 raw milk collection centers, 57 milk trucks, 62 traditional livestock farms, and 31 industrial livestock farms. Additionally, 69 samples of pasteurized and sterilized milk produced by nine milk factory brands were collected from supermarkets in North Khorasan Province. The sampling was conducted during the summer and autumn seasons. Before collecting the samples, all necessary materials and equipment were washed with detergent and sterilized by autoclaving. The collected milk samples were transported to the laboratory under sterile conditions and in an ice-cooled environment.
To prepare the samples, all chilled milk samples were initially centrifuged at 3500 rounds per min to obtain skimmed milk while completely removing the upper creamy layer. Subsequently, 100 µL of skimmed milk samples were extracted for AFM1 analysis.
In this experiment, an ELISA kit (Veratox, Neogen) with a sensitivity of 5 parts per trillion was employed to measure the concentration of AFM1 in milk samples. The test was performed according to the instructions provided by the manufacturer of the kit. Specifically, 50 μL of each standard (0, 250, 500, 1000, and 2000 ng/L) was added to 50 μL of skimmed milk samples in each well. Subsequently, 50 μL of conjugation and 50 μL of antibody were added to each well in sequence. To ensure thorough mixing of all contents in each well, the kit was manually moved several times in different directions. After allowing the kit to stand at room temperature for 10 min, the test results were recorded. After 10 min, the contents of the kit were removed, and each well was washed three times with distilled water. The kit was then inverted to drain the distilled water and ensure complete drying of the wells. Next, 100 μL of chromogen solution was added to each well, after which the kit was manually agitated in different directions to thoroughly mix the contents of each well. The kit was then placed in a dark environment for 5 min. After 5 min, 100 μL of stopping solution was added to each well, and the kit was agitated before measuring the light absorption using an ELISA reader at a wavelength of 650 nm. The concentrations of AFM1 were calculated by drawing a curve based on the recorded results. Additionally, the ELISA method was utilized in this study to identify positive samples for the presence of AFM1 in mastitis milk samples.
AFM1 rapid test kits are commonly used for the rapid detection of aflatoxin M1 in raw milk and milk products. In this study, all raw milk samples were assessed using AFM1 rapid test kits (Milkguard Rapid Test Kit for Aflatoxin M1, Kwinbon Biotech, China). According to the manufacturer’s instructions, the sensitivity of this kit was 0.1 parts per billion. The obtained results were analyzed using the SPSS software, version 22 and the Tukey test (analysis of variance) with a significance level of P<0.05.
Results
Over 6 months during the summer and autumn of 2023, a total of 189 raw milk samples and 69 samples of pasteurized and sterilized milk were collected from supermarkets in the province. The results showed that AFM1 was detected in 93.65% of raw milk samples and in 42% of pasteurized and sterilized milk samples, with concentrations ranging between 1-50 ng/L. The Mean±SD of AFM1 concentrations in raw and pasteurized milk samples varied across the cities of Bojnurd, Esfarayen, Shirvan, Jajarm, Mane and Samalqan, Garmeh, Faruj, and Raz and Jargalan, as well as different dairy factories, with the values of 17.31±21.72, 8.91±9.30, 30.70±80.50, 13.73±16.12, 17.69±21.16, 24.33±26.73, 18.09±13.35, 14.43±11.27, and 42.80±21.54 ng/L, respectively (Table 1).
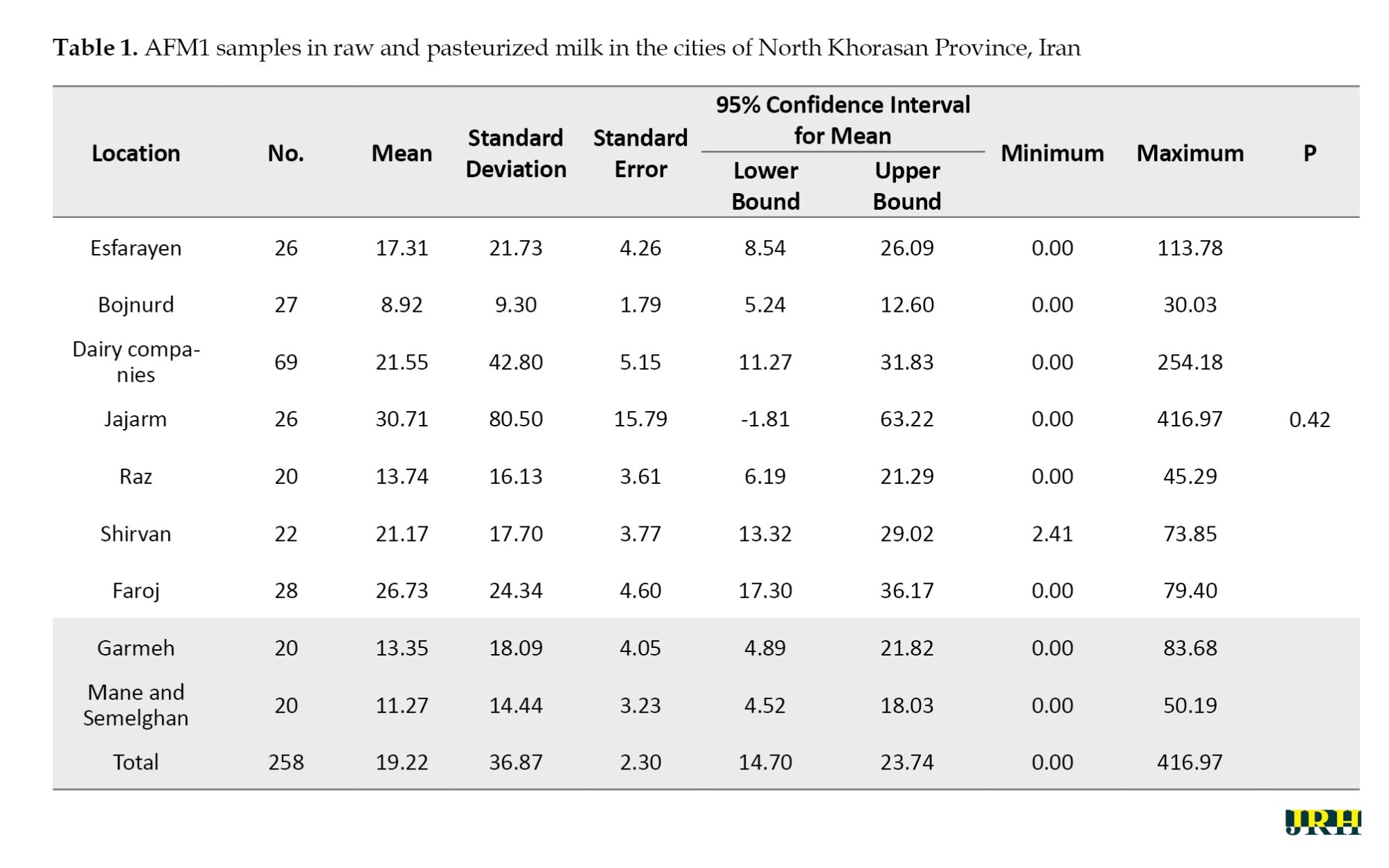
Bojnord had a higher mean level of AFM1 (8.91±9.30), while Jajarm had a lower mean AFM1 level (80.50±30.70). However, statistical analysis did not reveal any significant differences in AFM1 levels between the different cities or pasteurized milk (P=0.42).
The mean AFM1 concentration was lower in milk collection centers (11.40±18.6) and higher in industrial farms (26.92±21.47); however, this difference was not statistically significant (P=0.94).
The mean concentration of AFM1 in milk samples from North Khorasan Province (36.86±19.21) was lower than the European standard. However, in terms of relative frequency, the level of contamination in some cities, such as Esfrain and Jajarm, exceeded the national standard of Iran by 3.8%. However, all the milk samples analyzed in this study were below the permissible limit set by the Iranian Veterinary Organization (500 ng/L), as shown in Tables 2 and 3.

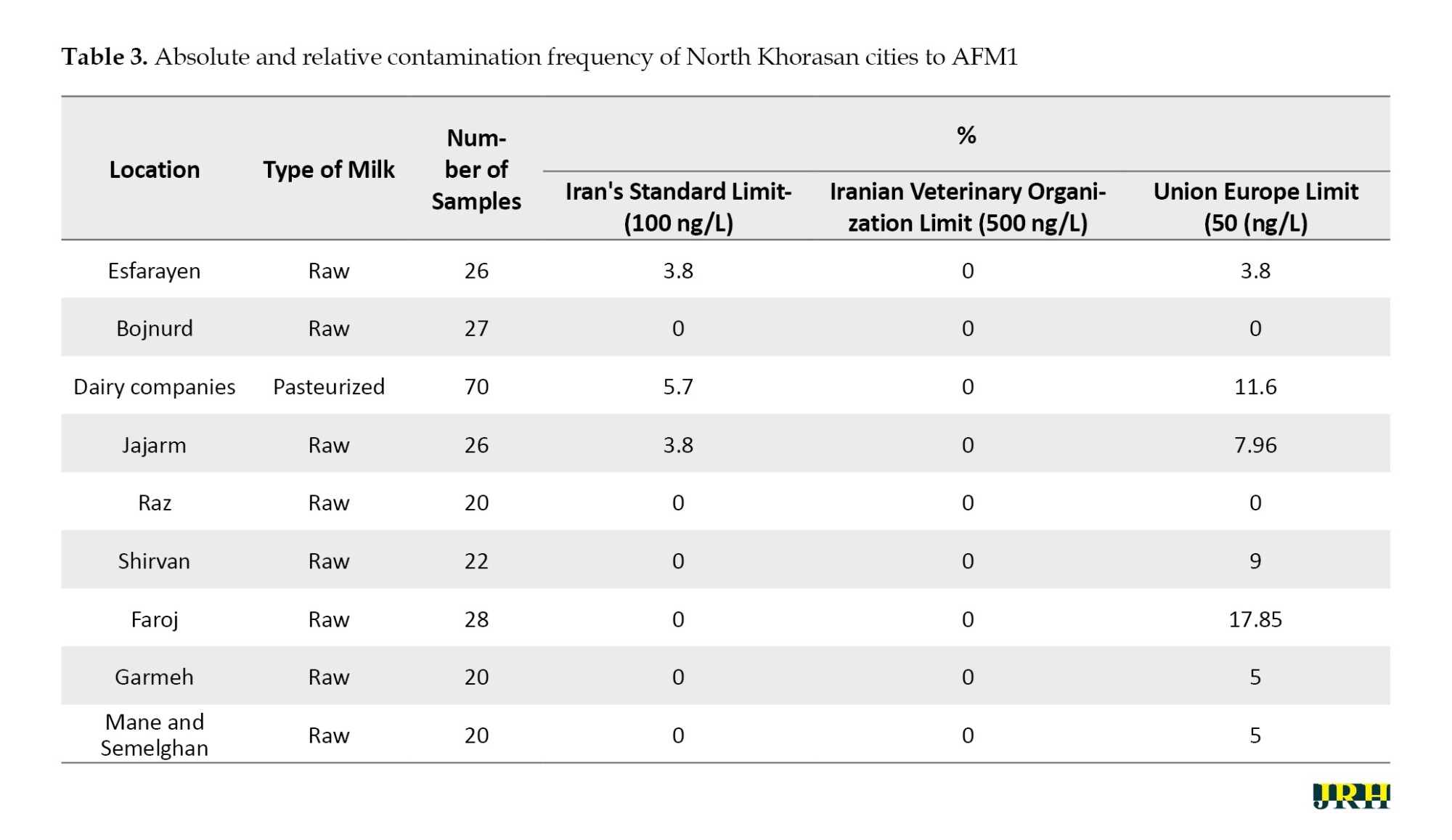
On the other hand, the average concentration of AFM1 in pasteurized and sterilized milk samples from the province was higher than both the European and Iranian standards, with a mean concentration of 42.8±21.54 ng/L. The relative frequency of samples exceeding the European and Iranian standards was 11.4% and 5.7%, respectively (Table 4). In this study, the sensitivity of the AFM1 rapid test kit for the detection of AFM1 in raw milk was evaluated and compared to the ELISA method. The results indicated that this kit lacked the necessary sensitivity to detect AFM1 in the concentration range of 50-500 ng/L; therefore, it did not exhibit sufficient efficiency for use in this study.

Discussion
The potential health risks associated with AFM1 in milk and dairy products are significant. In this study, 93.65% of raw milk and 87.14% of pasteurized and sterilized milk were contaminated with AFM1 in the range of 0-50 ng/L, which according to studies conducted in the European :union: (EU), are within the allowable limit of aflatoxin levels and do not have adverse effects on the health of adults [12]. The remaining milk that has higher contamination levels needs further analysis. To accurately assess and interpret these findings, it is essential to establish a recognized limit for AFM1 in the country. The existence of multiple standards across the country has complicated this analysis. Many researchers in Iran have compared and contrasted the EU’s standard limit of 50 ng/L [12] with the National Standard Organization’s limit of 100 ng/L [13]. These comparisons have raised concerns about the high levels of AFM1 in milk and underscore the need for a consistent and effective regulatory framework to safeguard public health [8-10, 14-21]. Iran Veterinary Organization is responsible for monitoring the health of raw milk and raw livestock products in Iran and has established a limit of 500 ng/L for AFM1 [22]. This has led to varying risk assessments by researchers. Similar double standards are also observed in other countries [23]. Accordingly, public health and economic factors have influenced the adoption of different standards, with major agricultural producers tending to have more lenient laws and importers having stricter ones. This highlights the need for global harmonization of standards to ensure the safety and quality of food products and to protect public health.
Switzerland has implemented the strictest restrictions on aflatoxin B1 by banning the use of peanut meal in livestock diets. However, this resolution may not be practical in countries that are major producers of such products [23]. These differences have led some countries to establish different limits for AFM1 based on age groups, such as children and adults, to prevent adverse effects (Table 5) [23]. This approach ensures that milk intended for children has lower AFM1 levels than that intended for adults. For instance, Brazil, which has set a limit of 500 ng/L for raw milk, has established a stricter limit of 10 ng/L for infant milk, which is more stringent than some European countries [23]. This approach can help minimize economic losses and protect the rights of consumers, particularly children who are more vulnerable to the harmful effects of aflatoxins. However, it also highlights the need for global harmonization of standards to ensure consistent protection of public health.

This study did not find a statistically significant difference in the mean AFM1 concentrations between raw and pasteurized milk samples collected from various cities in North Khorasan Province, Iran. Therefore, the overall mean AFM1 concentration in raw and pasteurized milk across the province can be considered a benchmark for the year 2022. This level of contamination is lower than that reported in some other studies conducted in different parts of the country. The study found that the relative abundance of AFM1 was 1.05% in raw milk and 5.7% in pasteurized milk, which exceeds the permissible limit of the national standard of Iran. Nevertheless, none of the samples in either raw or pasteurized milk exceeded the permissible limit set by the country’s veterinary organization (Table 4). Overall, the AFM1 contamination in raw and pasteurized milk in the province was considered to be in good condition and did not pose a significant health risk to the population, particularly adults. In a similar study, consumption of pasteurized milk and dairy products did not pose a risk of liver cancer to children and adults in Tehran City, Iran [18]. The study found that if the EU’s standard limit of 50 ng/L is applied, 6.34% of raw milk, and 11.04% of pasteurized and sterilized milk in the province exceed the limit, which is concerning given that children and elderly people are more susceptible to the harmful effects of AFM1 (Table 4). In a similar study conducted in Shahrood City, Iran, the level of AFM1 in school milk was reported to be 100% lower than the Iranian standard limit and 15.4% higher than the EU limit [8]. In another study, Sotoudeh et al. investigated the risk of carcinogenesis associated with AFM1 in milk. They calculated the AFM1 carcinogenic risk index, called the hazard index, in pasteurized milk samples from Kerman City and Rafsanjan City, Iran, which had an average AFM1 concentration of 30.9 ng/L. The results showed that milk consumption did not pose a health risk to adults, although the risk index increased for children, it did not pose a significant risk [5]. The study found that the average concentration of AFM1 in pasteurized milk was 21.54 ng/L, which is lower than the average concentration reported in the cities of Kerman and Rafsanjan, Iran, and is considered to be safe. In contrast, a study conducted in Qazvin City, Iran, reported average concentrations of 734 ng/L in raw milk and 268 ng/L in pasteurized milk, which are higher than the permissible limits of common standards. Therefore, a risk assessment for minors should be conducted to determine the potential health risks associated with these high levels of AFM1 [24]. To ensure consistency across the country, it is recommended to establish a single standard limit for children based on the level of AFM1 contamination in milk. Additionally, milk with lower levels of AFM1 contamination should be used to produce milk powder, cerlac, school milk, and other similar products. An analysis of research conducted in Iran over the past 20 years (2002 to 2022) indicates that the average concentration of AFM1 in raw milk samples has gradually decreased [5-28]. Over the past 20 years (2002 to 2022), the average concentration of AFM1 in raw milk samples has gradually decreased in Iran. The average concentrations in different regions were as follows: Tehran=207 ng/L, Ahvaz=155.91 ng/L, Gilan=123.2 ng/L, Mashhad=116 ng/L, Shiraz=112 ng/L, Babol=102 ng/L, Gorgan=76 ng/L, Isfahan=65 ng/L, Mazandaran=63.8 ng/L, Semnan=55.1 ng/L, Sanandaj=50.3 ng/L, Qazvin=38.8 ng/L, Kerman and Rafsanjan=30.9 ng/L, Yazd=22.07 ng/L, and North Khorasan=19.2 ng/L [5, 7, 9, 14, 18, 19, 25-28]. The high prevalence of aflatoxin in Gilan City, Iran, is attributed to the region’s high rainfall and humidity [9], while in Ahvaz City, the storage of animal feed is likely the cause of the high aflatoxin prevalence due to the growth of fungi and the production of AFM1 [14]. However, since 2001, the overall trend of decreasing average AFM1 concentrations can be attributed to the control measures implemented by relevant organizations. The Iranian Veterinary Organization, which is responsible for monitoring the health of raw milk, has implemented a systematic program for the control, supervision, and monitoring of raw milk during the past two decades.
Similarly, through subsidiary organizations, the university of medical sciences is responsible for monitoring dairy factories and has implemented similar measures. The combined efforts of these measures have had a positive effect in reducing the concentration of AFM1 in raw milk over the years. Therefore, it is necessary to continue with group cooperation to control and monitor milk and dairy products, especially in areas prone to fungal growth and AFM1 production due to weather and climate conditions.
Conclusion
The levels of AFM1 contamination in both raw and pasteurized/sterilized milk samples from North Khorasan Province, Iran, were within the permissible limits for public consumption. However, given that children are more sensitive to aflatoxins, there is a need for a single standard to ensure the safety of all consumers.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of North Khorasan University of Medical Sciences (Code: IR.NKUMS.REC.1400.162).
Funding
This study was financially supported by North Khorasan University of Medical Sciences (Research plan Code: 4000268).
Authors' contributions
Conceptualization, methodology, data analysis, and writing of the original draft: Mitra Salehi and Pezhman Bahari; Data collection and interpretation: Mahyar Sharifan and Touhid Valizadeh; Review, editing and final approval: Hamidreza Shoraka and Akbar Solati.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors express deepest gratitude to all those who have contributed to the creation and completion of this article. Their support, guidance, and expertise have been invaluable in shaping and refining the content.
References
ycotoxins are natural contaminants that can be found in food and agricultural products. They are created by molds and fungi, with certain types of fungi from the Aspergillus genus as the main culprits. Specifically, Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius are known to produce these toxins. According to the Food and Agriculture Organization (FDA), around 25% of agricultural products worldwide are affected by mycotoxin contamination [1]. Fungi tend to thrive in hot and humid environments and can produce mycotoxins. Among the mycotoxins identified in nature, at least 18 types of aflatoxins are known, with aflatoxin B1, B2, G1, and G2 being particularly significant. When animals consume feed contaminated with aflatoxin B1, the toxin is metabolized in the liver and converted into aflatoxin M1 (AFM1). This mycotoxin is then excreted in milk and can harm human organs, particularly the digestive system and liver. Depending on its concentration and duration, AFM1 can cause mutagenesis, carcinogenesis, and immune system suppression [2]. Meanwhile, AFM1 resists common heat treatment methods like pasteurization, sterilization, and autoclaving [3]. AFM1’s high resistance to freezing and fermentation makes it a concern in producing raw animal products like yogurt, buttermilk, and cheese, which can be made using contaminated milk [4]. Although children are more susceptible to the health risks associated with AFM1-contaminated milk, dairy products are also important sources of nutrients essential for human growth and well-being, especially in children [5]. The International Agency for Research on Cancer recently classified aflatoxin M1 as a Group 1 carcinogen, highlighting the significant health risks this mycotoxin poses [6]. To safeguard the safety and health of human societies, government institutions must conduct rigorous monitoring of food and livestock products. To mitigate the adverse effects of AFM1, many countries and international organizations have established maximum permissible limits for this mycotoxin in milk and dairy products. Various chromatography techniques, including thin-layer chromatography, liquid chromatography, high-performance liquid chromatography, and enzyme-linked immunosorbent assay (ELISA), are available for detecting and analyzing AFM1 in food, milk, and dairy products. ELISA is a particularly advantageous method, offering reduced test times, simple sample preparation and extraction, low cost, and high sensitivity [7]. Several studies have investigated the levels of AFM1 in raw and pasteurized milk in Iran, including in North Khorasan Province, Iran; however, a comprehensive study of this issue has yet to be conducted in the region [7-10]. Given that traditional livestock management practices are common in the province and may not prioritize health standards, it is necessary to implement specific management measures and prevention programs. Therefore, this study assesses the levels of AFM1 in raw, pasteurized, and sterilized milk produced in the cities within North Khorasan Province, Iran.
Methods
This study is a descriptive-analytical investigation conducted in North Khorasan Province, Iran. The minimum sample size was calculated using the method described by Karimi et al. in 2007, considering a contamination rate of 60% (P=0.6), a confidence level of 95%, and an acceptable error value of 5%, total sample size calculated was 258 [11] (Equation 1):

The samples were divided into two groups as follows: Raw and pasteurized milk. The sampling method in this study is multi-stage. First, we consider each city as a stratum, and sampling is done from all strata. In the next stage of sampling, the cluster sampling method was used, each industrial livestock unit was considered a cluster, and each unit of milk collection center and milk tanker was taken as a cluster. In addition, each traditional livestock was considered a cluster. Based on the number of traditional livestock in each village, the number of samples was determined. A total of 189 raw milk samples were collected randomly in 6 months from various sources in the cities of Bojnurd, Esfarayen, Shirvan, Jajarm, Mane and Samalqan, Garmeh, Faruj, and Raz and Jargalan. This study was conducted in the summer and autumn of 2022. These seasons are suitable for the growth of molds and fungi. The samples were obtained from 39 raw milk collection centers, 57 milk trucks, 62 traditional livestock farms, and 31 industrial livestock farms. Additionally, 69 samples of pasteurized and sterilized milk produced by nine milk factory brands were collected from supermarkets in North Khorasan Province. The sampling was conducted during the summer and autumn seasons. Before collecting the samples, all necessary materials and equipment were washed with detergent and sterilized by autoclaving. The collected milk samples were transported to the laboratory under sterile conditions and in an ice-cooled environment.
To prepare the samples, all chilled milk samples were initially centrifuged at 3500 rounds per min to obtain skimmed milk while completely removing the upper creamy layer. Subsequently, 100 µL of skimmed milk samples were extracted for AFM1 analysis.
In this experiment, an ELISA kit (Veratox, Neogen) with a sensitivity of 5 parts per trillion was employed to measure the concentration of AFM1 in milk samples. The test was performed according to the instructions provided by the manufacturer of the kit. Specifically, 50 μL of each standard (0, 250, 500, 1000, and 2000 ng/L) was added to 50 μL of skimmed milk samples in each well. Subsequently, 50 μL of conjugation and 50 μL of antibody were added to each well in sequence. To ensure thorough mixing of all contents in each well, the kit was manually moved several times in different directions. After allowing the kit to stand at room temperature for 10 min, the test results were recorded. After 10 min, the contents of the kit were removed, and each well was washed three times with distilled water. The kit was then inverted to drain the distilled water and ensure complete drying of the wells. Next, 100 μL of chromogen solution was added to each well, after which the kit was manually agitated in different directions to thoroughly mix the contents of each well. The kit was then placed in a dark environment for 5 min. After 5 min, 100 μL of stopping solution was added to each well, and the kit was agitated before measuring the light absorption using an ELISA reader at a wavelength of 650 nm. The concentrations of AFM1 were calculated by drawing a curve based on the recorded results. Additionally, the ELISA method was utilized in this study to identify positive samples for the presence of AFM1 in mastitis milk samples.
AFM1 rapid test kits are commonly used for the rapid detection of aflatoxin M1 in raw milk and milk products. In this study, all raw milk samples were assessed using AFM1 rapid test kits (Milkguard Rapid Test Kit for Aflatoxin M1, Kwinbon Biotech, China). According to the manufacturer’s instructions, the sensitivity of this kit was 0.1 parts per billion. The obtained results were analyzed using the SPSS software, version 22 and the Tukey test (analysis of variance) with a significance level of P<0.05.
Results
Over 6 months during the summer and autumn of 2023, a total of 189 raw milk samples and 69 samples of pasteurized and sterilized milk were collected from supermarkets in the province. The results showed that AFM1 was detected in 93.65% of raw milk samples and in 42% of pasteurized and sterilized milk samples, with concentrations ranging between 1-50 ng/L. The Mean±SD of AFM1 concentrations in raw and pasteurized milk samples varied across the cities of Bojnurd, Esfarayen, Shirvan, Jajarm, Mane and Samalqan, Garmeh, Faruj, and Raz and Jargalan, as well as different dairy factories, with the values of 17.31±21.72, 8.91±9.30, 30.70±80.50, 13.73±16.12, 17.69±21.16, 24.33±26.73, 18.09±13.35, 14.43±11.27, and 42.80±21.54 ng/L, respectively (Table 1).

Bojnord had a higher mean level of AFM1 (8.91±9.30), while Jajarm had a lower mean AFM1 level (80.50±30.70). However, statistical analysis did not reveal any significant differences in AFM1 levels between the different cities or pasteurized milk (P=0.42).
The mean AFM1 concentration was lower in milk collection centers (11.40±18.6) and higher in industrial farms (26.92±21.47); however, this difference was not statistically significant (P=0.94).
The mean concentration of AFM1 in milk samples from North Khorasan Province (36.86±19.21) was lower than the European standard. However, in terms of relative frequency, the level of contamination in some cities, such as Esfrain and Jajarm, exceeded the national standard of Iran by 3.8%. However, all the milk samples analyzed in this study were below the permissible limit set by the Iranian Veterinary Organization (500 ng/L), as shown in Tables 2 and 3.


On the other hand, the average concentration of AFM1 in pasteurized and sterilized milk samples from the province was higher than both the European and Iranian standards, with a mean concentration of 42.8±21.54 ng/L. The relative frequency of samples exceeding the European and Iranian standards was 11.4% and 5.7%, respectively (Table 4). In this study, the sensitivity of the AFM1 rapid test kit for the detection of AFM1 in raw milk was evaluated and compared to the ELISA method. The results indicated that this kit lacked the necessary sensitivity to detect AFM1 in the concentration range of 50-500 ng/L; therefore, it did not exhibit sufficient efficiency for use in this study.

Discussion
The potential health risks associated with AFM1 in milk and dairy products are significant. In this study, 93.65% of raw milk and 87.14% of pasteurized and sterilized milk were contaminated with AFM1 in the range of 0-50 ng/L, which according to studies conducted in the European :union: (EU), are within the allowable limit of aflatoxin levels and do not have adverse effects on the health of adults [12]. The remaining milk that has higher contamination levels needs further analysis. To accurately assess and interpret these findings, it is essential to establish a recognized limit for AFM1 in the country. The existence of multiple standards across the country has complicated this analysis. Many researchers in Iran have compared and contrasted the EU’s standard limit of 50 ng/L [12] with the National Standard Organization’s limit of 100 ng/L [13]. These comparisons have raised concerns about the high levels of AFM1 in milk and underscore the need for a consistent and effective regulatory framework to safeguard public health [8-10, 14-21]. Iran Veterinary Organization is responsible for monitoring the health of raw milk and raw livestock products in Iran and has established a limit of 500 ng/L for AFM1 [22]. This has led to varying risk assessments by researchers. Similar double standards are also observed in other countries [23]. Accordingly, public health and economic factors have influenced the adoption of different standards, with major agricultural producers tending to have more lenient laws and importers having stricter ones. This highlights the need for global harmonization of standards to ensure the safety and quality of food products and to protect public health.
Switzerland has implemented the strictest restrictions on aflatoxin B1 by banning the use of peanut meal in livestock diets. However, this resolution may not be practical in countries that are major producers of such products [23]. These differences have led some countries to establish different limits for AFM1 based on age groups, such as children and adults, to prevent adverse effects (Table 5) [23]. This approach ensures that milk intended for children has lower AFM1 levels than that intended for adults. For instance, Brazil, which has set a limit of 500 ng/L for raw milk, has established a stricter limit of 10 ng/L for infant milk, which is more stringent than some European countries [23]. This approach can help minimize economic losses and protect the rights of consumers, particularly children who are more vulnerable to the harmful effects of aflatoxins. However, it also highlights the need for global harmonization of standards to ensure consistent protection of public health.

This study did not find a statistically significant difference in the mean AFM1 concentrations between raw and pasteurized milk samples collected from various cities in North Khorasan Province, Iran. Therefore, the overall mean AFM1 concentration in raw and pasteurized milk across the province can be considered a benchmark for the year 2022. This level of contamination is lower than that reported in some other studies conducted in different parts of the country. The study found that the relative abundance of AFM1 was 1.05% in raw milk and 5.7% in pasteurized milk, which exceeds the permissible limit of the national standard of Iran. Nevertheless, none of the samples in either raw or pasteurized milk exceeded the permissible limit set by the country’s veterinary organization (Table 4). Overall, the AFM1 contamination in raw and pasteurized milk in the province was considered to be in good condition and did not pose a significant health risk to the population, particularly adults. In a similar study, consumption of pasteurized milk and dairy products did not pose a risk of liver cancer to children and adults in Tehran City, Iran [18]. The study found that if the EU’s standard limit of 50 ng/L is applied, 6.34% of raw milk, and 11.04% of pasteurized and sterilized milk in the province exceed the limit, which is concerning given that children and elderly people are more susceptible to the harmful effects of AFM1 (Table 4). In a similar study conducted in Shahrood City, Iran, the level of AFM1 in school milk was reported to be 100% lower than the Iranian standard limit and 15.4% higher than the EU limit [8]. In another study, Sotoudeh et al. investigated the risk of carcinogenesis associated with AFM1 in milk. They calculated the AFM1 carcinogenic risk index, called the hazard index, in pasteurized milk samples from Kerman City and Rafsanjan City, Iran, which had an average AFM1 concentration of 30.9 ng/L. The results showed that milk consumption did not pose a health risk to adults, although the risk index increased for children, it did not pose a significant risk [5]. The study found that the average concentration of AFM1 in pasteurized milk was 21.54 ng/L, which is lower than the average concentration reported in the cities of Kerman and Rafsanjan, Iran, and is considered to be safe. In contrast, a study conducted in Qazvin City, Iran, reported average concentrations of 734 ng/L in raw milk and 268 ng/L in pasteurized milk, which are higher than the permissible limits of common standards. Therefore, a risk assessment for minors should be conducted to determine the potential health risks associated with these high levels of AFM1 [24]. To ensure consistency across the country, it is recommended to establish a single standard limit for children based on the level of AFM1 contamination in milk. Additionally, milk with lower levels of AFM1 contamination should be used to produce milk powder, cerlac, school milk, and other similar products. An analysis of research conducted in Iran over the past 20 years (2002 to 2022) indicates that the average concentration of AFM1 in raw milk samples has gradually decreased [5-28]. Over the past 20 years (2002 to 2022), the average concentration of AFM1 in raw milk samples has gradually decreased in Iran. The average concentrations in different regions were as follows: Tehran=207 ng/L, Ahvaz=155.91 ng/L, Gilan=123.2 ng/L, Mashhad=116 ng/L, Shiraz=112 ng/L, Babol=102 ng/L, Gorgan=76 ng/L, Isfahan=65 ng/L, Mazandaran=63.8 ng/L, Semnan=55.1 ng/L, Sanandaj=50.3 ng/L, Qazvin=38.8 ng/L, Kerman and Rafsanjan=30.9 ng/L, Yazd=22.07 ng/L, and North Khorasan=19.2 ng/L [5, 7, 9, 14, 18, 19, 25-28]. The high prevalence of aflatoxin in Gilan City, Iran, is attributed to the region’s high rainfall and humidity [9], while in Ahvaz City, the storage of animal feed is likely the cause of the high aflatoxin prevalence due to the growth of fungi and the production of AFM1 [14]. However, since 2001, the overall trend of decreasing average AFM1 concentrations can be attributed to the control measures implemented by relevant organizations. The Iranian Veterinary Organization, which is responsible for monitoring the health of raw milk, has implemented a systematic program for the control, supervision, and monitoring of raw milk during the past two decades.
Similarly, through subsidiary organizations, the university of medical sciences is responsible for monitoring dairy factories and has implemented similar measures. The combined efforts of these measures have had a positive effect in reducing the concentration of AFM1 in raw milk over the years. Therefore, it is necessary to continue with group cooperation to control and monitor milk and dairy products, especially in areas prone to fungal growth and AFM1 production due to weather and climate conditions.
Conclusion
The levels of AFM1 contamination in both raw and pasteurized/sterilized milk samples from North Khorasan Province, Iran, were within the permissible limits for public consumption. However, given that children are more sensitive to aflatoxins, there is a need for a single standard to ensure the safety of all consumers.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of North Khorasan University of Medical Sciences (Code: IR.NKUMS.REC.1400.162).
Funding
This study was financially supported by North Khorasan University of Medical Sciences (Research plan Code: 4000268).
Authors' contributions
Conceptualization, methodology, data analysis, and writing of the original draft: Mitra Salehi and Pezhman Bahari; Data collection and interpretation: Mahyar Sharifan and Touhid Valizadeh; Review, editing and final approval: Hamidreza Shoraka and Akbar Solati.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors express deepest gratitude to all those who have contributed to the creation and completion of this article. Their support, guidance, and expertise have been invaluable in shaping and refining the content.
References
- Deshpande SS. Handbook of food toxicology. Taylor & Francis. 2017. [Link]
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. The American Journal of Clinical Nutrition. 2004; 80(5):1106-22. [DOI:10.1093/ajcn/80.5.1106] [PMID]
- Dimitrieska-Stojković E, Stojanovska-Dimzoska B, Ilievska G, Uzunov R, Stojković G, Hajrulai-Musliu Z, et al. Assessment of aflatoxin contamination in raw milk and feed in Macedonia during 2013. Food Control. 2016; 59:201-6. [DOI:10.1016/j.foodcont.2015.05.019]
- El Marnissi B, Belkhou R, Morgavi DP, Bennani L, Boudra H. Occurrence of aflatoxin M1 in raw milk collected from traditional dairies in Morocco. Food Chem Toxicol. 2012; 50(8):2819-21. [DOI:10.1016/j.fct.2012.05.031] [PMID]
- Sotoodeh L, Dini A, Rezaeian L, Esmaeili A, Asgarian A. [Evaluation of aflatoxin M1 contamination in pasteurized milk in Kerman and Rafsanjan cities in 2019: A descriptive study (Persian)]. Journal of Rafsanjan University of Medical Sciences. 2021; 19 (11):1163-78. [DOI:10.29252/jrums.19.11.1163]
- International Agency for Research on Cancer (IARC). List of classifications by cancer sites with sufficient or limited evidence in humans, IARC Monographs Volumes 1-135a. Geneva: WHO; 2023. [Link]
- Yahyaraeyat R, Shokri H, Khosravi AR, Torabi S. [Evaluation of the contamination of aflatoxin M1 level in raw milk samples by ELISA method in Yazd province (Persian)]. Journal of Veterinary Research. 2017; 72(3):313-21. [DOI: 10.22059/JVR.2017.222337.2551]
- Moeinian K, Rastgoo T. Seasonal variation of aflatoxin M1 contamination in raw, pasteurized and school milk in Shahrood, Iran. Journal of Mazandaran University of Medical Sciences. 2014; 24:19-28. [Link]
- Najafian M, Najafian B. [Investigation of the level of aflatoxin M1 in milk samples of Gilan dairy factories using ELISA (Persian)]. Journal of Microbial World. 2015; 8(3):248-53. [Link]
- Golestan L, Rahimi K. [Determination of aflatoxin M1 level in raw milk of east Mazandaran retails (Persian)]. Journal of Food Hygiene. 2019; 9(34):85-91. [Link]
- Karimi G, Hassanzadeh M, Teimuri M, Nazari F, Nili A. Aflatoxin M1 contamination in pasteurized milk in Mashhad, Iran. Iranian Journal of Pharmaceutical Sciences. 2007; 3(3):153-6. [DOI:10.22037/ijps.v3.41008]
- Food and Agriculture Organization (FAO). Worldwide regulations for mycotoxins in food and feed in 2003. Rome: FAO; 2005. [Link]
- Islamic Republic of Iran Institute of Standards and Industrial Research of Iran (ISIRI). Food & feed-mycotoxins-maximum tolerated level. Institute of Standards and Industrial Research of Iran. 2010; 5925(amendment No.1). [Link]
- Ghariby H, Takdastan A, Neisi AK, Rezazadeh H, Kuhpaee H. [Investigating aflatoxin M1 contamination in buffalo milk using immunoassay (Persian)]. Journal of Mazandaran University of Medical Sciences. 2017; 26(145):248-56. [Link]
- Sanatgar A, Aghazadeh M. [Aflatoxin M1 contamination of raw milk in Khoy city (Persian)]. Veterinary Researches & Biological Products. 2017; 30(4):147-54. [Link]
- Barami AR, Elmi MRP, Irani M. [Contamination levels of aflatoxin M1 in bulk raw milk of Chaloos and Ramsar (Persian)]. Food Hygiene. 2011; 4(1):53-61. [Link]
- Hashemi M. A survey of aflatoxin M1 in cow milk in southern Iran. Journal of Food and Drug Analysis. 2016; 24(4):888-93. [DOI:10.1016/j.jfda.2016.05.002] [PMID]
- Abyaneha HK, Bahonara A, Nooria N, HassanYazdanpanahb, AliAbadi MHS. Exposure to aflatoxin M1 through milk consumption in Tehran population, Iran. Iranian Journal of Pharmaceutical Research. 2018; 18(1):506-14. [DOI: 10.22037/ijpr.2019.1100764] [PMID]
- Moeinian K, Yaghmaeian K, Ghorbani R. [Aflatoxin M1 concentration in raw milk produced in the cities of Semnan province - Iran (Persian)]. Koomesh. 2014; 15(2):176-81. [Link]
- Movassagh MH, Adinehvand S. [Study of aflatoxin M1 level in the collected raw cow milk from milk collection centers in Tabriz (Persian). Journal of Food Hygiene. 2013; 3(4): 49-55. [Link]
- Safavizadeh V, Mojkar M. A survey on the occurrence of aflatoxin M1 in raw milk samples in Bafq and Bahabad, city. Journal of Food Safety and Hygiene. 2019; 5(3):175- 8. [Link]
- Institute of Standards and Industrial Research of Iran (ISIRI). Food & Feed- Maximum limit of heavy metals. ISIRI: Karaj; 2011. [Link]
- Galvano F, Galofaro V, Galvano G. Occurrence and stability of aflatoxin M1 in milk and milk products: a worldwide review. Journal of Food Protection. 1996; 59: 1079-90. [DOI: 10.4315/0362-028X-59.10.1079] [PMID]
- Norian R, Pourfarzaneh A, Mashatian F. [Determination of aflatoxin M1 in raw milk produced in Qazvin Province by ELISA and HPLC (Persian)]. Journal of Food Microbiology. 2014; 1(3):7-13. [Link]
- Sadeghi E, Mohammadi M, Sadeghi M, Mohammadi R. [Systematic review study of aflatoxin M1 level in raw, pasteurized and UHT milk in Iran (Persian)]. Iranian Journal of Nutrition Sciences & Food Technology. 2013; 7: 599- 612. [Link]
- Mohsanzadeh M, Bisjerdi S. [Determination of aflatoxin M1 level in UHT milk by ELISA (Persian)]. Paper presented at: Proceedings of the 4th Biotechnology National Congress. 14 June 2005. Kerman; Iran. [Link]
- Abdali F, Zare M, Abbasi A, Berizi E. Aflatoxin M1 occurrence in local dairy products in Shiraz, Southern Iran. International Journal of Nutrition Sciences. 2020; 5(3):142-7. [Link]
- Rahimi E, A S, M J. Occurrence of aflatoxin M1 in raw, pasteurized and UHT milk commercial in Isfahan. Food Science. 317-320. [DOI:10.1007/s12571-009-0028-9]
- Gholi pour M, Karim Zadeh L, Ali Nia F, Babaee Z. Determination of aflatoxin M1 in milk processed in Mazandaran dairy factories, 2011. Journal of Mazandaran University of Medical Sciences. 2012; 22:39-46. [Link]
Type of Study: Orginal Article |
Subject:
● Disease Control
Received: 2023/07/29 | Accepted: 2023/10/17 | Published: 2024/07/1
Received: 2023/07/29 | Accepted: 2023/10/17 | Published: 2024/07/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |