Volume 15, Issue 4 (Jul & Aug 2025)
J Research Health 2025, 15(4): 393-402 |
Back to browse issues page
Ethics code: IR.MUBAM.REC.1400.019
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fazli Z, Mohammadian F, Ghorbanpour N, Firouzeh N. The Effect of Stress Caused by Occupational Noise on the Salivary Cortisol Levels of Weaving Industry Workers. J Research Health 2025; 15 (4) :393-402
URL: http://jrh.gmu.ac.ir/article-1-2569-en.html
URL: http://jrh.gmu.ac.ir/article-1-2569-en.html
1- Department of Occupational Health Engineering and Safety, School of Public Health and Safety, Bam University of Medical Sciences, Bam, Iran. & Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Environmental Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3- Department of Occupational Health Engineering and Safety, School of Public Health and Safety, Bam University of Medical Sciences, Bam, Iran.
4- Vector-Borne Diseases Research Center, North Khorasan University of Medical Sciences, Bojnurd, Iran. ,Nimafirouzeh4@gmail.com
2- Environmental Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3- Department of Occupational Health Engineering and Safety, School of Public Health and Safety, Bam University of Medical Sciences, Bam, Iran.
4- Vector-Borne Diseases Research Center, North Khorasan University of Medical Sciences, Bojnurd, Iran. ,
Full-Text [PDF 637 kb]
(552 Downloads)
| Abstract (HTML) (3335 Views)
Full-Text: (623 Views)
Introduction
Humans’ physical, mental, and social health is strongly influenced by their work environment [1, 2]. Among the many factors that impact this environ-ment, noise has become a major concern in recent years [3]. The primary noise source in industrial settings stems from the machinery’s moving com-ponents [4]. These noise resources produce acoustic emissions that have been identified as sources of environmental stress [5]. According to the International Labor Organization (ILO), approximately 3.3 billion individuals are employed globally [6]. According to the centers for disease control (CDC), approximately 22 million workers in the United States are exposed to harmful noise each year [7]. In Iran, approximately 15% of workshops with more than 10 employees are exposed to damaging noise, which affects approximately 20% of the workers [8].
Additionally, it can reduce human performance, productivity, and concentration and increase the risk of accidents and errors. In the workplace, noise is any unwanted or undesirable sound that can adversely affect workers’ health, safety, and productivity. It can include continuous noise from ma-chinery, intermittent noise from tools or processes, or even sudden, loud noises that startle workers [9]. The tolerance threshold for noise in workers is the maximum level of noise exposure that workers can safely endure without experiencing significant negative impacts on their health and well-being [10].
Occupational exposure to above 85 dB for eight hours a day can cause high blood pressure, decreased work performance, sleep problems, confusion, and stress [11]. Stress has numerous negative effects on the body. When humans experience stress, the body releases stress hormones such as corti-sol and adrenaline. This stress response includes physical and mental reactions aimed at helping the body cope with the perceived threat or challenge. However, prolonged stress can result in chronic elevation of cortisol levels, which can cause a range of health concerns. The adverse effects of stress on the body include increased blood pressure, increased heart rate, gastrointestinal disturbances, muscle weakness, decreased immune system func-tion, increased risk for heart disease, further stimulation of stress hormone secretion, and increased risk for depression and anxiety [12].
Several reliable indicators are at experts’ disposal for measuring and determining stress levels. Cortisol is a glucocorticoid hormone produced by the adrenal glands. This hormone can be easily detected in blood or urine samples, making it a practical and reliable stress indicator. Among the nonaudi-tory effects of noise exposure are increased stress hormone levels, which disrupt hormonal rhythms by activating the sympathetic adrenal medullary and the hypothalamic pituitary adrenal (HPA) axes. In this regard, cortisol can be an excellent indicator for evaluating stress levels and performance of the HPA axis due to noise exposure [13]. Various factors can affect the level of cortisol in the human body. Some of these factors were as follows:
1) Stress; cortisol levels increase when individuals experience stressful situations such as work pressure, family issues, illness, poor nutrition, or unfa-vorable conditions; 2) Physical activity; exercise, and physical activity can also increase cortisol levels; 3) Sleep; getting enough sleep at the right time can positively affect cortisol levels; 4) Diseases; certain diseases, such as cancer, Addison disease, Cushing syndrome, and manic-depressive illness, can cause an increase in cortisol levels in the body; 5) Drugs; certain medications, such as corticosteroids, can also increase cortisol levels.
In addition, a combination of factors, along with individual genetics, can also impact cortisol levels [14, 15].
Stress indicators such as cortisol are widely used to study the biological effects of noise exposure [12]. The body’s response to stress leads to the secretion of high levels of cortisol. Because this hormone is responsible for several stress-related changes in the body, it is also called a stress hor-mone [12, 16, 17]. Several studies have investigated the impact of noise on blood cortisol levels. While some studies have revealed a significant difference in cortisol levels before and after noise exposure, others have produced conflicting results [16, 18, 19]. In contrast, in some other studies, this relationship was not confirmed [20, 21].
Noise exposure can act as a stressor and adversely impact the body’s secretion of stress system mediators, including free hormones such as cortico-tropin, catecholamines, norepinephrine, epinephrine, and cortisol. These hormones can exert destructive effects on the body [22]. Calming music and other stress-reducing activities can decrease cortisol levels, while loud or external noise can increase them. Studies have demonstrated that individu-als residing in noisy environments have a greater level of cortisol [23]. Before this investigation, no scientific findings addressed the correlations among noise exposure, occupational stress and cortisol levels in workers within the weaving industry.
Given the multiple sources of noise in this industry and the high noise exposure experienced by workers, it is essential to examine their stress levels. Therefore, this study aims to investigate the impact of occupational noise stress on the salivary cortisol levels of textile industry workers.
Methods
Study participants
This quasi-experimental study was conducted in a weaving factory in southeast Iran. In the textile hall, many machines, such as blenders, autorollers, double layer, double warp, and finishers, made a lot of noise when turned on. In this study, the studied population was selected from the same exposure groups in one textile hall that used the overnight facilities provided by the factory management. Seventy-two workers had this initial condition. Using the Cochran formula (z: 1.96; p=q=0.5; d: 0.05), the sample size was calculated to be 61 cases.
The exclusion criteria included hearing impairment, headache, head surgery, mental shock (in the last 6 months), cardiovascular disease, metabolic disorders, diabetes, work experience of less than 1 year, and smoking. Eight people were excluded from the study due to these conditions. Therefore, 53 workers were selected from the textile hall.
Data collection methods
In the first step, written informed consent was obtained from all workers. Next, they completed a structured checklist containing individual demographic information, such as gender, age, weight, height, and work experience.
In this study, workers’ stress was investigated subjectively and objectively, including using a standard occupational stress questionnaire and the level of salivary cortisol hormone as an indicator of stress. The purpose of using the standard occupational stress questionnaire was to measure all stressful factors and correctly judge the factors affecting the changes in the cortisol hormone level as an objective indicator in this study.
The Osipow occupational stress questionnaire (OJSQ) is a widely used tool to assess occupational stress and its impact on individuals. The primary purpose of this questionnaire is to measure the stress experienced by people in their work environment and provide insight into the factors influencing that stress [24].
The study samples were people who worked in a textile hall doing the same activity and spent their rest day in the boarding house. After the workers filled out the occupational stress questionnaire, they were examined on two consecutive days (one working day and one rest day) and two times a day (morning and evening). Choosing one group of people and comparing them with themselves was to control most factors involved in causing stress, including physiological differences, nutrition, activity, disease, drug use, and genetics.
Therefore, in this situation, it seems that the only variable that affects the stress level is the noise exposure.
Osipow job stress questionnaire
To measure the level of stress, there are several stress indices, including the international stress questionnaire, the personnel stress questionnaire, Cohen and Williamson’s perceived stress scale, the job strain scale, and OJSQ. Among them, OJSQ is useful for measuring and comparing individual job stress levels and identifying the main factors exacerbating job stress [25, 26].
The OJSQ is a widely used tool to assess occupational stress and its impact on individuals. The primary purpose of the OJSQ is to measure the stress experienced by individuals in their work environment and to provide insight into the factors contributing to that stress. Overall, the OJSQ is a valuable tool for researchers, organizations, and individuals to assess and understand occupational stress levels, which are important for promoting employee well-being, improving job performance, and maintaining a healthy work environment [27].
This study used the Persian version of the standard OJSQ. This questionnaire was first developed in 1987 by Osipow et al. and its reliability was confirmed by a re-test method with a 89% Cronbach α. The Persian version of this tool has been used in various studies in Iran, and its validity and reliability have been confirmed [28]. The first part of this questionnaire is related to demographic characteristics, including age, gender, type of job, marital status, employment status, and shift work. The other part of the tool contains 60 questions related to job stress and is designed based on job role, consisting of 6 subgroups, each containing 10 questions.
The six dimensions included the following: Workload, incompetence, duality, role range, role responsibility, and physical environment. All questionnaire items were designed based on a 5-point Likert scale ranging from 1 (never) to 5 (always). The overall stress level was estimated according to the total score of 60 questions. Based on the scores obtained, the total stress level was divided into 5 categories: Stress below level (60 to 109), normal level (110 to 159), mild level (160 to 209), moderate level (210 to 259), and severe level (260 to 300). In this questionnaire, the stress level of each subgroup of stressors is considered in three categories: Mild (scores less than 23), moderate (scores between 23 and 36) and severe (scores more than 36) [24].
Noise monitoring
The CEL-231 sound dosimeter (Cel Instruments, Ltd., Bedford, England) and its calibrator (CEL-110/2) were used to measure and monitor the noise. Our assessment began with a comprehensive visit to the textile factory, where we meticulously checked the noise sources and the workers’ exposure. This detailed inspection was crucial for ensuring the accuracy of the sound dosimeter device, which was connected to the workers’ hearing area (clothing collar, according to the NSI S12.10-2019 standard recommendations and ISO 9612:2009 standard). Sound dosimetry was performed for 8 hours [19, 29]. To evaluate the employees’ exposure to noise, Equation 1 was used [30].
Dosimetry was done on two consecutive days, working day (in the textile hall) and rest day (in the boarding house).
Equation 1:
1. Leq=10log10[(Dose/100)]
Saliva collection
Saliva samples were collected on the morning and afternoon of two consecutive days: A working day exposed to noise in the textile hall, and a rest day exposed to normal noise in the boarding house [31].
Participants were asked to place 2 mL of their saliva sample into a sterile vial upon waking up and before brushing, eating, drinking between 6:00 AM and 7:00 AM (the start of their shift) and at 2:00 PM (end of working time).
Four saliva samples were collected from each participant (2 saliva samples at work and two at rest). The collected samples were transferred to the laboratory and stored in a freezer at -18 °C until analysis. Salivary cortisol was determined using the electrochemiluminescence immunoassay, which uses an Elecsys™ immunoassay analyzer and the cobas™ salivary kit (Roche Diagnostics GmbH, Mannheim, Germany [32].
Statistical analysis
The Kolmogorov‒Smirnov test was used to check the normality of the data distribution. All the statistical analyses were performed using SPSS software, version 16.
The t-test and regression analysis were applied in the present survey and P<0.05 were considered to indicate a statistically significant difference.
Results
Participants’ demographic information is presented in Table 1. The Kolmogorov-Smirnov test results indicated that the data exhibited a normal distribution. There was no significant difference between the male and female participants regarding mean age or work experience (P>0.05). The results also showed that all participants were more exposed to noise on working days than the permissible occupational limits (85 dB) (Table 1).
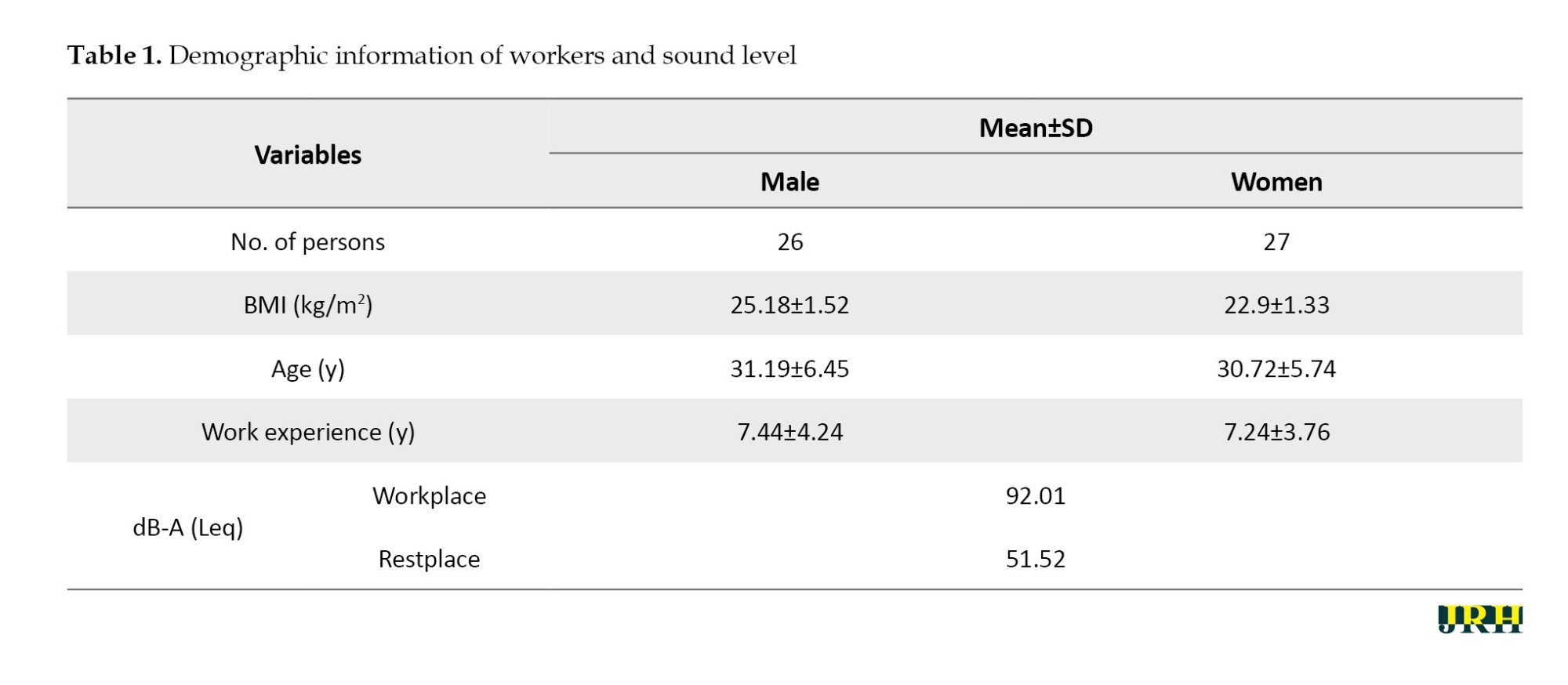
Our data confirmed that the salivary cortisol level was higher on working days than during rest for both men and women (Table 2). However, the independent t-test analysis showed no significant difference between males and females in any comparisons. On the other hand, the paired t-test data revealed a significant difference among participants when comparing cortisol levels on work and rest days (P<0.05).
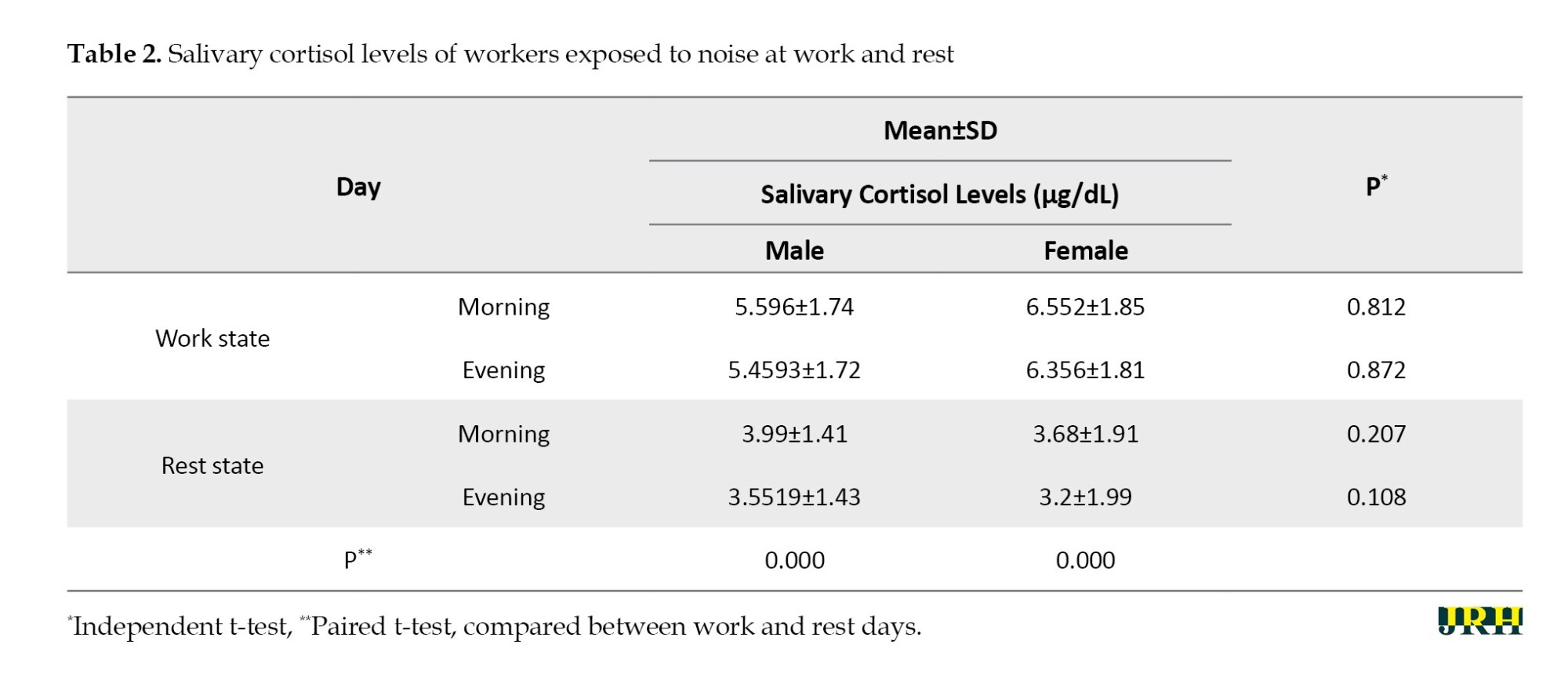
Table 3 provides a clear picture. Out of the six dimensions of stress assessments, only the workload score significantly differed between the two groups (P=0.006). However, it is important to note that despite this difference, participants experienced mild stress, with a final questionnaire score of 163.3 for men and 160.4 for women, falling within the mild stress range of 160 to 209 (P>0.05). This finding is further supported by the fact that the total stress scores were not statistically differ-ent between the studied groups, reinforcing the robustness of our research.
Discussion
Our findings reveal a significant difference in salivary cortisol levels among the groups during morning and evening shifts on working days and during noise exposure. This difference in cortisol levels, also reported in other studies [19, 33, 34], underscores the importance of our research. For instance, a study on car assembly workers demonstrated that while moderate noise exposure did not disrupt cortisol secretion, high noise exposure significantly increased cortisol levels [35].
Other studies examining the effect of noise exposure on cortisol showed that exposure to low noise levels does not affect changes in cortisol levels [36]. This study supports the hypothesis that the psychological stress caused by exposure to workplace noise leads to impaired cortisol regulation. The average score of the answers obtained from the occupational stress questionnaire for all participants, all stress subgroups, was at the moderate level, except for role responsibility, which was at the mild level with an average score of 22.09. In general, it can be concluded that all participants are at the mild level (160 to 209) with an average score of 161.82. Therefore, different factors did not greatly impact people’s stress.
Our study’s results revealed no significant difference between the changes in cortisol levels in the morning and evening in the two sex groups, aligning with the findings of other studies. However, what sets our research apart is the significant correlation we found between changes in cortisol concentrations and exposure to workplace noise in both men and women, mirroring the results of studies by Baudin et al. and Evrard et al [37, 38]. These findings suggest a direct relationship between cortisol changes and workplace noise exposure, potentially through noise disturbance, stress, and sensitivity. This finding underscores the role of noise as a nonspecific stressor that triggers the autonomic nervous system and the endocrine pathway [38].
The use of salivary cortisol in this study, instead of blood or cortisol in urine, is due to its ease of measurement, reliability, and noninvasiveness [39, 40]. However, it is crucial to acknowledge that cortisol secretion can be influenced by a multitude of factors such as season, time and week, sampling day, sex, age, BMI, physical activity, alcohol consumption, smoking, medication, high food intake, sleep quality, occupational activity, hormonal contraception and pregnancy [41, 42]. We used paired t-tests to control for the effects of confounding variables, such as genetics, nutrition, disease, drug use, and activity. Furthermore, saliva sampling was conducted twice during the working day, with the same two tests repeated on the resting day. These parameters may affect the association between workplace noise exposure and cortisol secretion. In the present study, confounders were controlled as much as possible by determining both sexes’ inclusion criteria and complications.
Several similar reports exist in this field. For example, in 2021, Dehaghi et al. conducted a study on noise-induced stress that measured salivary cortisol levels and found a significant correlation between exposure to industrial noise and salivary cortisol secretion [35]. Also, Barbaresco et al. showed that with increasing noise lev-els, salivary cortisol levels also increase during the day. After adjusting for age, sex, smoking status and time, with a 1 dB increase in Leq, salivary cortisol levels in-creased by 0.25 nmol/L between the morning and evening [43].
However, Stokholm et al. found no significant correlation between salivary cortisol levels and recent or long-term occupational exposure to occupational noise (70 to 120 dB A) outside working hours [44].
Conclusion
In conclusion, our study supports the notion that both men and women, regardless of their gender, exposed to industrial noise experienced similar changes in salivary cortisol levels, indicating that noise exposure induced job stress universally. Additionally, our findings revealed that exposure to workplace noise can alter salivary cortisol levels. The results suggest the need for further investigation into the variations in salivary cortisol levels between morning and evening, causal relationships, and the biological mechanisms underlying the impact of noise exposure on regulating the HPA axis.
Strengths and limitations
This study had several strong points. In this study, first, the studied population’s cortisol levels were compared with their own levels rather than those of a control group. The subjects were examined over two consecutive days (one working day and one rest day) and twice during the work shift (morning and evening) to control for various factors influencing stress, including physiological differences, nutrition, activity, disease, drug use, and genetics. Second, considering the circadian cycle, the subjects’ cortisol levels were measured in the morning and afternoon. Third, before assessing the subjects’ cortisol levels, they were surveyed using a standard occupa-tional stress questionnaire to identify various stress-causing factors. The study aimed to examine stress levels in response to noise, so exploring different occupational factors was considered important. Before this research, similar studies had yet to investigate this aspect. Fourth, the study focused on individuals who had similar exposure to noise in both their work and rest environments. Last, until this study, no scientific reports had been published on the relationships between noise expo-sure, occupational stress, and cortisol levels in textile workers.
One limitation of our investigation is transporting saliva samples to the laboratory promptly after collection.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Bam University of Medical Sciences, Bam, Iran (Code: IR.NKUMS.REC.1400.117). An adequate level of confidentiality, the right to withdraw from the study at any stage, the ability to communicate research objectives honestly and transparently, the ability to obtain written consent from the subjects before the study, and the ability to use the data only for the research were among the ethical considerations of the study.
Funding
The research was financially supported by Bam University of Medical Sciences, Bam, Iran (Grant No.: IR.MUBAM.REC.1400.019).
Authors' contributions
Conceptualization, Zohreh Fazli; Methodology: Farough Mohammadian; Software: Najmeh Ghorbanpour, and Nima Firouzeh; Writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors want to acknowledge all staff from the Department of Occupational Health Engineering and Safety, School of Public Health and Safety, Bam University of Medical Sciences, Bam, Iran.
References
Humans’ physical, mental, and social health is strongly influenced by their work environment [1, 2]. Among the many factors that impact this environ-ment, noise has become a major concern in recent years [3]. The primary noise source in industrial settings stems from the machinery’s moving com-ponents [4]. These noise resources produce acoustic emissions that have been identified as sources of environmental stress [5]. According to the International Labor Organization (ILO), approximately 3.3 billion individuals are employed globally [6]. According to the centers for disease control (CDC), approximately 22 million workers in the United States are exposed to harmful noise each year [7]. In Iran, approximately 15% of workshops with more than 10 employees are exposed to damaging noise, which affects approximately 20% of the workers [8].
Additionally, it can reduce human performance, productivity, and concentration and increase the risk of accidents and errors. In the workplace, noise is any unwanted or undesirable sound that can adversely affect workers’ health, safety, and productivity. It can include continuous noise from ma-chinery, intermittent noise from tools or processes, or even sudden, loud noises that startle workers [9]. The tolerance threshold for noise in workers is the maximum level of noise exposure that workers can safely endure without experiencing significant negative impacts on their health and well-being [10].
Occupational exposure to above 85 dB for eight hours a day can cause high blood pressure, decreased work performance, sleep problems, confusion, and stress [11]. Stress has numerous negative effects on the body. When humans experience stress, the body releases stress hormones such as corti-sol and adrenaline. This stress response includes physical and mental reactions aimed at helping the body cope with the perceived threat or challenge. However, prolonged stress can result in chronic elevation of cortisol levels, which can cause a range of health concerns. The adverse effects of stress on the body include increased blood pressure, increased heart rate, gastrointestinal disturbances, muscle weakness, decreased immune system func-tion, increased risk for heart disease, further stimulation of stress hormone secretion, and increased risk for depression and anxiety [12].
Several reliable indicators are at experts’ disposal for measuring and determining stress levels. Cortisol is a glucocorticoid hormone produced by the adrenal glands. This hormone can be easily detected in blood or urine samples, making it a practical and reliable stress indicator. Among the nonaudi-tory effects of noise exposure are increased stress hormone levels, which disrupt hormonal rhythms by activating the sympathetic adrenal medullary and the hypothalamic pituitary adrenal (HPA) axes. In this regard, cortisol can be an excellent indicator for evaluating stress levels and performance of the HPA axis due to noise exposure [13]. Various factors can affect the level of cortisol in the human body. Some of these factors were as follows:
1) Stress; cortisol levels increase when individuals experience stressful situations such as work pressure, family issues, illness, poor nutrition, or unfa-vorable conditions; 2) Physical activity; exercise, and physical activity can also increase cortisol levels; 3) Sleep; getting enough sleep at the right time can positively affect cortisol levels; 4) Diseases; certain diseases, such as cancer, Addison disease, Cushing syndrome, and manic-depressive illness, can cause an increase in cortisol levels in the body; 5) Drugs; certain medications, such as corticosteroids, can also increase cortisol levels.
In addition, a combination of factors, along with individual genetics, can also impact cortisol levels [14, 15].
Stress indicators such as cortisol are widely used to study the biological effects of noise exposure [12]. The body’s response to stress leads to the secretion of high levels of cortisol. Because this hormone is responsible for several stress-related changes in the body, it is also called a stress hor-mone [12, 16, 17]. Several studies have investigated the impact of noise on blood cortisol levels. While some studies have revealed a significant difference in cortisol levels before and after noise exposure, others have produced conflicting results [16, 18, 19]. In contrast, in some other studies, this relationship was not confirmed [20, 21].
Noise exposure can act as a stressor and adversely impact the body’s secretion of stress system mediators, including free hormones such as cortico-tropin, catecholamines, norepinephrine, epinephrine, and cortisol. These hormones can exert destructive effects on the body [22]. Calming music and other stress-reducing activities can decrease cortisol levels, while loud or external noise can increase them. Studies have demonstrated that individu-als residing in noisy environments have a greater level of cortisol [23]. Before this investigation, no scientific findings addressed the correlations among noise exposure, occupational stress and cortisol levels in workers within the weaving industry.
Given the multiple sources of noise in this industry and the high noise exposure experienced by workers, it is essential to examine their stress levels. Therefore, this study aims to investigate the impact of occupational noise stress on the salivary cortisol levels of textile industry workers.
Methods
Study participants
This quasi-experimental study was conducted in a weaving factory in southeast Iran. In the textile hall, many machines, such as blenders, autorollers, double layer, double warp, and finishers, made a lot of noise when turned on. In this study, the studied population was selected from the same exposure groups in one textile hall that used the overnight facilities provided by the factory management. Seventy-two workers had this initial condition. Using the Cochran formula (z: 1.96; p=q=0.5; d: 0.05), the sample size was calculated to be 61 cases.
The exclusion criteria included hearing impairment, headache, head surgery, mental shock (in the last 6 months), cardiovascular disease, metabolic disorders, diabetes, work experience of less than 1 year, and smoking. Eight people were excluded from the study due to these conditions. Therefore, 53 workers were selected from the textile hall.
Data collection methods
In the first step, written informed consent was obtained from all workers. Next, they completed a structured checklist containing individual demographic information, such as gender, age, weight, height, and work experience.
In this study, workers’ stress was investigated subjectively and objectively, including using a standard occupational stress questionnaire and the level of salivary cortisol hormone as an indicator of stress. The purpose of using the standard occupational stress questionnaire was to measure all stressful factors and correctly judge the factors affecting the changes in the cortisol hormone level as an objective indicator in this study.
The Osipow occupational stress questionnaire (OJSQ) is a widely used tool to assess occupational stress and its impact on individuals. The primary purpose of this questionnaire is to measure the stress experienced by people in their work environment and provide insight into the factors influencing that stress [24].
The study samples were people who worked in a textile hall doing the same activity and spent their rest day in the boarding house. After the workers filled out the occupational stress questionnaire, they were examined on two consecutive days (one working day and one rest day) and two times a day (morning and evening). Choosing one group of people and comparing them with themselves was to control most factors involved in causing stress, including physiological differences, nutrition, activity, disease, drug use, and genetics.
Therefore, in this situation, it seems that the only variable that affects the stress level is the noise exposure.
Osipow job stress questionnaire
To measure the level of stress, there are several stress indices, including the international stress questionnaire, the personnel stress questionnaire, Cohen and Williamson’s perceived stress scale, the job strain scale, and OJSQ. Among them, OJSQ is useful for measuring and comparing individual job stress levels and identifying the main factors exacerbating job stress [25, 26].
The OJSQ is a widely used tool to assess occupational stress and its impact on individuals. The primary purpose of the OJSQ is to measure the stress experienced by individuals in their work environment and to provide insight into the factors contributing to that stress. Overall, the OJSQ is a valuable tool for researchers, organizations, and individuals to assess and understand occupational stress levels, which are important for promoting employee well-being, improving job performance, and maintaining a healthy work environment [27].
This study used the Persian version of the standard OJSQ. This questionnaire was first developed in 1987 by Osipow et al. and its reliability was confirmed by a re-test method with a 89% Cronbach α. The Persian version of this tool has been used in various studies in Iran, and its validity and reliability have been confirmed [28]. The first part of this questionnaire is related to demographic characteristics, including age, gender, type of job, marital status, employment status, and shift work. The other part of the tool contains 60 questions related to job stress and is designed based on job role, consisting of 6 subgroups, each containing 10 questions.
The six dimensions included the following: Workload, incompetence, duality, role range, role responsibility, and physical environment. All questionnaire items were designed based on a 5-point Likert scale ranging from 1 (never) to 5 (always). The overall stress level was estimated according to the total score of 60 questions. Based on the scores obtained, the total stress level was divided into 5 categories: Stress below level (60 to 109), normal level (110 to 159), mild level (160 to 209), moderate level (210 to 259), and severe level (260 to 300). In this questionnaire, the stress level of each subgroup of stressors is considered in three categories: Mild (scores less than 23), moderate (scores between 23 and 36) and severe (scores more than 36) [24].
Noise monitoring
The CEL-231 sound dosimeter (Cel Instruments, Ltd., Bedford, England) and its calibrator (CEL-110/2) were used to measure and monitor the noise. Our assessment began with a comprehensive visit to the textile factory, where we meticulously checked the noise sources and the workers’ exposure. This detailed inspection was crucial for ensuring the accuracy of the sound dosimeter device, which was connected to the workers’ hearing area (clothing collar, according to the NSI S12.10-2019 standard recommendations and ISO 9612:2009 standard). Sound dosimetry was performed for 8 hours [19, 29]. To evaluate the employees’ exposure to noise, Equation 1 was used [30].
Dosimetry was done on two consecutive days, working day (in the textile hall) and rest day (in the boarding house).
Equation 1:
1. Leq=10log10[(Dose/100)]
Saliva collection
Saliva samples were collected on the morning and afternoon of two consecutive days: A working day exposed to noise in the textile hall, and a rest day exposed to normal noise in the boarding house [31].
Participants were asked to place 2 mL of their saliva sample into a sterile vial upon waking up and before brushing, eating, drinking between 6:00 AM and 7:00 AM (the start of their shift) and at 2:00 PM (end of working time).
Four saliva samples were collected from each participant (2 saliva samples at work and two at rest). The collected samples were transferred to the laboratory and stored in a freezer at -18 °C until analysis. Salivary cortisol was determined using the electrochemiluminescence immunoassay, which uses an Elecsys™ immunoassay analyzer and the cobas™ salivary kit (Roche Diagnostics GmbH, Mannheim, Germany [32].
Statistical analysis
The Kolmogorov‒Smirnov test was used to check the normality of the data distribution. All the statistical analyses were performed using SPSS software, version 16.
The t-test and regression analysis were applied in the present survey and P<0.05 were considered to indicate a statistically significant difference.
Results
Participants’ demographic information is presented in Table 1. The Kolmogorov-Smirnov test results indicated that the data exhibited a normal distribution. There was no significant difference between the male and female participants regarding mean age or work experience (P>0.05). The results also showed that all participants were more exposed to noise on working days than the permissible occupational limits (85 dB) (Table 1).

Our data confirmed that the salivary cortisol level was higher on working days than during rest for both men and women (Table 2). However, the independent t-test analysis showed no significant difference between males and females in any comparisons. On the other hand, the paired t-test data revealed a significant difference among participants when comparing cortisol levels on work and rest days (P<0.05).

Table 3 provides a clear picture. Out of the six dimensions of stress assessments, only the workload score significantly differed between the two groups (P=0.006). However, it is important to note that despite this difference, participants experienced mild stress, with a final questionnaire score of 163.3 for men and 160.4 for women, falling within the mild stress range of 160 to 209 (P>0.05). This finding is further supported by the fact that the total stress scores were not statistically differ-ent between the studied groups, reinforcing the robustness of our research.

Discussion
Our findings reveal a significant difference in salivary cortisol levels among the groups during morning and evening shifts on working days and during noise exposure. This difference in cortisol levels, also reported in other studies [19, 33, 34], underscores the importance of our research. For instance, a study on car assembly workers demonstrated that while moderate noise exposure did not disrupt cortisol secretion, high noise exposure significantly increased cortisol levels [35].
Other studies examining the effect of noise exposure on cortisol showed that exposure to low noise levels does not affect changes in cortisol levels [36]. This study supports the hypothesis that the psychological stress caused by exposure to workplace noise leads to impaired cortisol regulation. The average score of the answers obtained from the occupational stress questionnaire for all participants, all stress subgroups, was at the moderate level, except for role responsibility, which was at the mild level with an average score of 22.09. In general, it can be concluded that all participants are at the mild level (160 to 209) with an average score of 161.82. Therefore, different factors did not greatly impact people’s stress.
Our study’s results revealed no significant difference between the changes in cortisol levels in the morning and evening in the two sex groups, aligning with the findings of other studies. However, what sets our research apart is the significant correlation we found between changes in cortisol concentrations and exposure to workplace noise in both men and women, mirroring the results of studies by Baudin et al. and Evrard et al [37, 38]. These findings suggest a direct relationship between cortisol changes and workplace noise exposure, potentially through noise disturbance, stress, and sensitivity. This finding underscores the role of noise as a nonspecific stressor that triggers the autonomic nervous system and the endocrine pathway [38].
The use of salivary cortisol in this study, instead of blood or cortisol in urine, is due to its ease of measurement, reliability, and noninvasiveness [39, 40]. However, it is crucial to acknowledge that cortisol secretion can be influenced by a multitude of factors such as season, time and week, sampling day, sex, age, BMI, physical activity, alcohol consumption, smoking, medication, high food intake, sleep quality, occupational activity, hormonal contraception and pregnancy [41, 42]. We used paired t-tests to control for the effects of confounding variables, such as genetics, nutrition, disease, drug use, and activity. Furthermore, saliva sampling was conducted twice during the working day, with the same two tests repeated on the resting day. These parameters may affect the association between workplace noise exposure and cortisol secretion. In the present study, confounders were controlled as much as possible by determining both sexes’ inclusion criteria and complications.
Several similar reports exist in this field. For example, in 2021, Dehaghi et al. conducted a study on noise-induced stress that measured salivary cortisol levels and found a significant correlation between exposure to industrial noise and salivary cortisol secretion [35]. Also, Barbaresco et al. showed that with increasing noise lev-els, salivary cortisol levels also increase during the day. After adjusting for age, sex, smoking status and time, with a 1 dB increase in Leq, salivary cortisol levels in-creased by 0.25 nmol/L between the morning and evening [43].
However, Stokholm et al. found no significant correlation between salivary cortisol levels and recent or long-term occupational exposure to occupational noise (70 to 120 dB A) outside working hours [44].
Conclusion
In conclusion, our study supports the notion that both men and women, regardless of their gender, exposed to industrial noise experienced similar changes in salivary cortisol levels, indicating that noise exposure induced job stress universally. Additionally, our findings revealed that exposure to workplace noise can alter salivary cortisol levels. The results suggest the need for further investigation into the variations in salivary cortisol levels between morning and evening, causal relationships, and the biological mechanisms underlying the impact of noise exposure on regulating the HPA axis.
Strengths and limitations
This study had several strong points. In this study, first, the studied population’s cortisol levels were compared with their own levels rather than those of a control group. The subjects were examined over two consecutive days (one working day and one rest day) and twice during the work shift (morning and evening) to control for various factors influencing stress, including physiological differences, nutrition, activity, disease, drug use, and genetics. Second, considering the circadian cycle, the subjects’ cortisol levels were measured in the morning and afternoon. Third, before assessing the subjects’ cortisol levels, they were surveyed using a standard occupa-tional stress questionnaire to identify various stress-causing factors. The study aimed to examine stress levels in response to noise, so exploring different occupational factors was considered important. Before this research, similar studies had yet to investigate this aspect. Fourth, the study focused on individuals who had similar exposure to noise in both their work and rest environments. Last, until this study, no scientific reports had been published on the relationships between noise expo-sure, occupational stress, and cortisol levels in textile workers.
One limitation of our investigation is transporting saliva samples to the laboratory promptly after collection.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Bam University of Medical Sciences, Bam, Iran (Code: IR.NKUMS.REC.1400.117). An adequate level of confidentiality, the right to withdraw from the study at any stage, the ability to communicate research objectives honestly and transparently, the ability to obtain written consent from the subjects before the study, and the ability to use the data only for the research were among the ethical considerations of the study.
Funding
The research was financially supported by Bam University of Medical Sciences, Bam, Iran (Grant No.: IR.MUBAM.REC.1400.019).
Authors' contributions
Conceptualization, Zohreh Fazli; Methodology: Farough Mohammadian; Software: Najmeh Ghorbanpour, and Nima Firouzeh; Writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors want to acknowledge all staff from the Department of Occupational Health Engineering and Safety, School of Public Health and Safety, Bam University of Medical Sciences, Bam, Iran.
References
- Kuhn S, Milasi S, Yoon S. World employment social outlook: Trends 2018. Geneva: International Labour Office; 2018. [Link]
- Zendehdel R, Fazli Z, Rezazadeh Azari M. Neurological risk assessment of co-exposure to heavy metals (chromium and nickel) in chromium-electroplating workers. Work. 2019; 63(3):355-60. [DOI:10.3233/WOR-192941] [PMID]
- Anees MM, Qasim M, Bashir A. Physiological and physical impact of noise pollution on environment. Earth Science Pakistan. 2017; 1(1):08-11. [DOI:10.26480/esp.01.2017.08.10]
- Yasar SS, Komut O, Yasar M, Fidan MS. Noise as a physical risk factor in furniture industry machines. BioResources. 2024; 19(2):2017-28. [DOI:10.15376/biores.19.2.2017-2028]
- Monazzam MR, Golmohammadi R, Nourollahi M, Momen BF. Assessment and control design for steam vent noise in an oil refinery. 2011; 11(1):14-9. [Link]
- Kharchani D, Haripitak R, Burirat C. The ILO's contribution to improving the welfare of workers globally, especially in Asia and Africa. Journal of Asian Multicultural Research for Social Sciences Study. 2021; 2(2):15-9. [DOI:10.47616/jamrsss.v2i2.130]
- Themann CL, Masterson EA. Occupational noise exposure: A review of its effects, epidemiology, and impact with recommendations for reducing its burden. The Journal of the Acoustical Society of America. 2019; 146(5):3879. [DOI:10.1121/1.5134465] [PMID]
- Oreyzi HR, Amiri M, Bahadorian P. Noise psychological effect in Isfahan hospitals environment. Journal of Mazandaran University of Medical Sciences. 2012; 21(86):182-91. [Link]
- Nassiri P, Heidari HR, Khadem M, Rahimifard H, Rostami E. Assessment of noise annoyance and its effects on healthcare staff based on sound pressure level and annoyance scale. International Journal of Occupational Hygiene. 2014; 6(1):23-30. [Link]
- Hon CY, Tchernikov I, Fairclough C, Behar A. Case study in a working environment highlighting the divergence between noise intensity and workers' perception towards noise. International Journal of Environmental Research and Public Health. 2020; 17(17):6122. [DOI:10.3390/ijerph17176122] [PMID] [PMCID]
- Farhang Dehghan S, Monazzam M R, Nassiri P, Haghighi Kafash Z, Jahangiri M. The assessment of noise exposure and noise annoyance at a petrochemical company. Journal of Health and Safety at Work. 2013; 3(3):11-24. [Link]
- McLaren E, Maxwell-Armstrong C. Noise pollution on an acute surgical ward. Annals of the Royal College of Surgeons of England. 2008; 90(2):136-9. [DOI:10.1308/003588408X261582] [PMID] [PMCID]
- Tahara Y, Sakurai K, Ando T. Influence of chewing and clenching on salivary cortisol levels as an indicator of stress. Journal of Prosthodontics. 2007; 16(2):129-35. [DOI:10.1111/j.1532-849X.2007.00178.x] [PMID]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews. Endocrinology. 2009; 5(7):374-81. [DOI:10.1038/nrendo.2009.106] [PMID]
- Strohmayer EA, Krakoff LR. Glucocorticoids and cardiovascular risk factors. Endocrinology and Metabolism Clinics of North America. 2011; 40(2):409-17. [DOI:10.1016/j.ecl.2011.01.011] [PMID]
- Risdiana H, Martiana T, Indriani D. Comparison cortisol level before and after exposed noise. International Journal of Advanced Engineering, Management and Science. 2016; 2(6):637-9. [Link]
- Khazali H. Effect of stress induced by epinephrine and cortisol injection on orexin secretion in male rats fed with different levels of their energy requirement. Iranian Journal of Endocrinology and Metabolism. 2012; 14(3):283-8. [Link]
- Fouladi DB, Nassiri P, Monazzam EM, Farahani S, Hassanzadeh G, Hoseini M. Industrial noise exposure and salivary cortisol in blue collar industrial workers. Noise & Health. 2012; 14(59):184-9. [DOI:10.4103/1463-1741.99894] [PMID]
- Zare S, Baneshi MR, Hemmatjo R, Ahmadi S, Omidvar M, Dehaghi BF. The effect of occupational noise exposure on serum cortisol concentration of night-shift industrial workers: A Field Study. Safety and Health at Work. 2019; 10(1):109-13. [DOI:10.1016/j.shaw.2018.07.002] [PMID] [PMCID]
- Michaud DS, Miller SM, Ferrarotto C, Konkle AT, Keith SE, Campbell KB. Waking levels of salivary biomarkers are altered following sleep in a lab with no further increase associated with simulated night-time noise exposure. Noise & Health. 2006; 8(30):30-9. [DOI:10.4103/1463-1741.32465] [PMID]
- Schwela DH. The new World Health Organization guidelines for community noise. Noise Control Engineering Journal. 2001; 49(4):193-8. [DOI:10.3397/1.2839659]
- Hahner S, Ross RJ, Arlt W, Bancos I, Burger-Stritt S, Torpy DJ, et al. Adrenal insufficiency. Nature Reviews. Disease Primers. 2021; 7(1):19. [DOI:10.1038/s41572-021-00252-7] [PMID]
- Kim HG, Cheon EJ, Bai DS, Lee YH, Koo BH. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investigation. 2018; 15(3):235-45. [DOI:10.30773/pi.2017.08.17] [PMID] [PMCID]
- Osipow SH, Spokane AR. Occupational stress inventory. Lutz, FL: Psychological Assessment Resources; 1987.
- J F Wilod Versprille L, J A E van de Loo A, Mackus M, Arnoldy L, A L Sulzer T, Vermeulen SA, et al. Development and validation of the immune status questionnaire (ISQ). International Journal of Environmental Research and Public Health. 2019; 16(23):4743. [DOI:10.3390/ijerph16234743] [PMID] [PMCID]
- Salinas CR, Webb HE. Occupational stress and coping mechanisms in crime scene personnel. Occupational Medicine. 2018; 68(4):239-45. [DOI:10.1093/occmed/kqy030] [PMID]
- Ahmadinejad P. Relationship between occupational stress, demographic characteristics, and job role. International Journal of Pharmaceutical Research. 2021; 13(1):1-6. [DOI:10.31838/ijpr/2021.13.01.743]
- Komeili-Sani M. The relationship between nurses' clinical competency and job stress in Ahvaz university hospital, 2013. Journal of Clinical Nursing and Midwifery. 2015; 4:39. [Link]
- American National Standards Institute. Quantities and procedures for description and measurement of environmental sound-part 4: Noise assessment and prediction of long-term community response. New York: American National Standards Institute; 2005. [Link]
- Abbasi M, Yazdanirad S, Mehri A, Madvari RF, Alizadeh A, Ghaljahi M, et al. Noise exposure and job stress–a structural equation model in textile industries. Archives of Acoustics. 2020; 45(4):601-11. [Link]
- Miller R, Stalder T, Jarczok M, Almeida DM, Badrick E, Bartels M, et al. Corrigendum to "The CIRCORT database: Reference ranges and seasonal changes in diurnal salivary cortisol derived from a meta-dataset comprised of 15 field studies" [PNEC 73C (2016) 16-23]. Psychoneuroendocrinology. 2017; 76:226-227. [DOI:10.1016/j.psyneuen.2016.11.038] [PMID]
- Stoffel M, Neubauer AB, Ditzen B. How to assess and interpret everyday life salivary cortisol measures: A tutorial on practical and statistical considerations. Psychoneuroendocrinology. 2021; 133:105391. [DOI:10.1016/j.psyneuen.2021.105391] [PMID]
- Lefèvre M, Carlier MC, Champelovier P, Lambert J, Laumon B, Evrard AS. Effects of aircraft noise exposure on saliva cortisol near airports in France. Occupational and Environmental Medicine. 2017; 74(8):612-8. [DOI:10.1136/oemed-2016-104208] [PMID]
- Strahler J, Skoluda N, Kappert MB, Nater UM. Simultaneous measurement of salivary cortisol and alpha-amylase: Application and recommendations. Neuroscience and Biobehavioral Reviews. 2017; 83:657-77. [DOI:10.1016/j.neubiorev.2017.08.015] [PMID]
- Fouladi Dehaghi B, Khademian F, Ahmadi Angali K. Non-auditory effects of industrial chronic noise exposure on workers; change in salivary cortisol pattern. Journal of Preventive Medicine and Hygiene. 2021; 61(4):E650-3. [DOI:10.15167/2421-4248/jpmh2020.61.4.1380] [PMID]
- Baroqah B, Sudjata RG, Irawan DJ. The benefits of stress relieving treatment in a Healing Forest Program: A pilot project at Ranca Upas, Ciwidey, West Java. Paper presented at: IOP Conference Series: Earth and Environmental Science. 1 November 2021; Bristol, England. [DOI:10.1088/1755-1315/918/1/012009]
- Baudin C, Lefèvre M, Selander J, Babisch W, Cadum E, Carlier MC, et al. Saliva cortisol in relation to aircraft noise exposure: Pooled-analysis results from seven European countries. Environmental Health. 2019; 18(1):102. [DOI:10.1186/s12940-019-0540-0] [PMID] [PMCID]
- Evrard AS, Lefèvre M, Champelovier P, Lambert J, Laumon B. Does aircraft noise exposure increase the risk of hypertension in the population living near airports in France? Occupational and Environmental Medicine. 2017; 74(2):123-9. [DOI:10.1136/oemed-2016-103648] [PMID]
- El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva - Are our assays good enough? Annals of Clinical Biochemistry. 2017; 54(3):308-22. [DOI:10.1177/0004563216687335] [PMID]
- Nunes LA, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochemia Medica. 2015; 25(2):177-92. [DOI:10.11613/BM.2015.018] [PMID] [PMCID]
- Milas G, Šupe-Domić D, Drmić-Hofman I, Rumora L, Klarić IM. Weather conditions: A neglected factor in human salivary cortisol research? International Journal of Biometeorology. 2018; 62(2):165-75. [DOI:10.1007/s00484-017-1436-8] [PMID]
- Bethge J, Fietz J, Razafimampiandra JC, Ruthsatz K, Dausmann KH. Season and reproductive activity influence cortisol levels in the Malagasy primate Lepilemur edwardsi. Journal of Experimental Zoology. 2022; 337(9-10):994-1001. [DOI:10.1002/jez.2658] [PMID]
- Barbaresco GQ, Reis AVP, Lopes GDR, Boaventura LP, Castro AF, Vilanova TCF, et al. Effects of environmental noise pollution on perceived stress and cortisol levels in street vendors. Journal of Toxicology and Environmental Health. 2019; 82(5):331-7. [DOI:10.1080/15287394.2019.1595239] [PMID]
- Stokholm ZA, Erlandsen M, Schlünssen V, Basinas I, Bonde JP, Peters S, et al. A quantitative general population job exposure matrix for occupational noise exposure. Annals of Work Exposures and Health. 2020; 64(6):604-13. [DOI:10.1093/annweh/wxaa034] [PMID]
Type of Study: Orginal Article |
Subject:
● Health Systems
Received: 2024/06/10 | Accepted: 2024/10/16 | Published: 2025/07/1
Received: 2024/06/10 | Accepted: 2024/10/16 | Published: 2025/07/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |